•To vote through the internet, go to http://www.envisionreports.com/NLNKwww.proxyvote.com to complete an electronic proxy card. You will be asked to provide the company number and control number from the Notice.
Your proxy, telephone or internet vote must be received by 11:59 p.m., EST,Eastern Time, on May 11, 201730, 2024 to be counted.
2
Beneficial Owner: Shares Registered in the Name of Broker or Bank
If you are a beneficial owner of shares registered in the name of your broker, bank, or other agent, you should have received a Notice containing voting instructions from that organization rather than from us. Simply follow the voting instructions in the Notice to ensure that your vote is counted. To vote in person at the Annual Meeting via live webcast, you must obtain a valid proxy from your broker, bank or other agent. Follow the instructions from your broker or bank included with these proxy materials, or contact your broker or bank to request a proxy form.
Internet proxy voting may be provided to allow you to vote your shares online, with procedures designed to ensure the authenticity and correctness of your proxy vote instructions. However, please be aware that you must bear any costs associated with your internet access, such as usage charges from internet access providers and telephone companies.
How many votes do I have?
On each matter to be voted upon, you have one vote for each share of common stock you own as of March 15, 2017.April 8, 2024. Common stock is the only class of voting securities currently outstanding and entitled to vote.
What happens if I do not vote?
Stockholder of Record: Shares Registered in Your Name
If you are a stockholder of record and do not vote (1) by completing and returning your proxy card, (2) by telephone, (3) through the internet or (4) in person at the Annual Meeting via live webcast, your shares will not be voted.
Beneficial Owner: Shares Registered in the Name of Broker or Bank
If you are a beneficial owner and do not instruct your broker, bank, or other agent how to vote your shares, the question of whether your broker or nominee will still be able to vote your shares depends on whether the New York Stock Exchange (NYSE) deems the particular proposal to be a “routine” matter. Brokers and nominees can use their discretion to vote “uninstructed” shares with respect to matters that are considered to be “routine,” but not with respect to “non-routine” matters. Under the rules and interpretations of the New York Stock Exchange, “non-routine” matters are matters that may substantially affect the rights or privileges of shareholders,stockholders, such as mergers, shareholderstockholder proposals, elections of directors (even if not contested), executive compensation (including any advisory shareholderstockholder votes on executive compensation and on the frequency of shareholderstockholder votes on executive compensation), and certain corporate governance proposals, even if management-supported. These rules apply to brokers holding our shares even though our common stock is traded on the NASDAQ Global Market. Accordingly, your broker or nominee may not vote your shares on ProposalsProposal No. 1 (Election of Directors), Proposal No. 2 (Advisory Vote on Compensation of Our Named Executive Officers), or 2 without your instructions,Proposal No. 3 (Frequency of Compensation Vote) but may vote your shares on Proposal 3No. 4 (Ratification of Selection of KPMG LLP as Our Independent Registered Public Accounting Firm) even in the absence of your instruction.
What if I return a proxy card or otherwise vote but do not make specific choices?
If you return a signed and dated proxy card or otherwise vote without marking voting selections, your shares will be voted, as applicable, “For” the election of the nominees for director, “For” the advisory approval of executive compensation, "One Year" for the frequency of future advisory votes on executive compensation, and “For” ratification of the selection, by the Audit Committee of our Board, of KPMG LLP as our independent registered public accounting firm for our fiscal year ending December 31, 2017.2024. If any other matter is properly presented at the meeting, your proxyholder (one of the individuals named on your proxy card) will vote your shares using his or her best judgment.
3
Who is paying for this proxy solicitation?
We will pay for the entire cost of soliciting proxies. In addition to these proxy materials, our directors and employees may also solicit proxies in person, by telephone or by other means of communication. Directors and employees will not be paid any additional compensation for soliciting proxies. We may also reimburse brokerage firms, banks and other agents for the cost of forwarding proxy materials to beneficial owners.
What does it mean if I receive more than one Notice?
If you receive more than oneNotice, your shares may be registered in more than one name or in different accounts. For example, you may own some shares directly as a stockholder of record and other shares through a broker, or you may own shares through more than one broker. Please follow the voting instructions on eachNotice to ensure that all of your shares are voted.
Can I change my vote after submitting my proxy?
Stockholder of Record: Shares Registered in Your Name
Yes. You can revoke your proxy at any time before the final vote at the Annual Meeting. If you are the record holder of your shares, you may revoke your proxy in any one of the following ways:
•You may submit another properly completed proxy card with a later date;
•You may grant a subsequent proxy by telephone or through the internet;
•You may send a timely written notice that you are revoking your proxy to NewLink Genetics Corporation’sthe Company’s Secretary at 2503 South Loop Drive, Suite 5100, Ames, IA 50010;4200 Marathon Boulevard #200, Austin, TX 78756; or
•You may attend the Annual Meeting and vote in person via live webcast (simply attending the meeting will not, by itself, revoke your proxy).
Your most current proxy card or telephone or internet proxy is the one that is counted.
Beneficial Owner: Shares Registered in the Name of Broker or Bank
If your shares are held by your broker or bank as a nominee or agent, you should follow the instructions provided by your broker or bank.
When are stockholder proposals due for next year’s annual meeting?
You are also advised to elect directors, votes “For,” “Withhold”review our Bylaws, which contain additional requirements regarding advance notice of stockholder proposals and broker non-votes and, with respect to other proposals, votes “For” and “Against,” abstentions and, if applicable, broker non-votes. Abstentions will be counted toward the vote total for Proposal Nos. 2 and 3, and will have the same effect as “Against” votes, but broker non-votes will have no effect on whether these proposals are approved. Broker non-votes will not be counted toward the vote total and will have no effect for Proposal No.1.director nominations.
4
What happens if I do not provide instructions on how to vote or if other matters are “broker non-votes”?presented for determination at the Annual Meeting?
If you are a beneficial owner ofas noted above you generally cannot vote your shares held in “street name” does not give instructions to thedirectly and must instead instruct your broker, trustee, bank or nominee holding the shares as to how to vote your shares using the voting instructions form provided by that intermediary. If you do not provide voting instructions, whether your shares can be voted by your broker, bank or nominee depends on matters deemed by the NYSE to be “non-routine,”type of item being considered.
•Non-Discretionary Items. If you do not provide voting instructions for any of the non-discretionary items at the Annual Meeting, your broker, bank or nominee cannot vote the shares. These unvotedyour shares, resulting in a “broker non-vote.” All items of business other than Proposal No. 4 (Ratification of Selection of KPMG LLP as Our Independent Registered Public Accounting Firm) are non-discretionary items. Shares constituting broker non-votes will be counted as “broker non-votes.”present for the purpose of determining a quorum at the Annual Meeting, but generally are not counted or deemed to be present in person or by proxy for the purpose of voting on any of the non-discretionary items.
•Discretionary Items. Even if you do not provide voting instructions, your broker, bank or nominee may vote in its discretion on Proposal No. 4 (Ratification of Selection of KPMG LLP as Our Independent Registered Public Accounting Firm) because it is a discretionary item.
What items are needed to approve each proposal?
being voted upon, how does the Board recommend that you vote, and what are the standards for determining whether an item has been approved?
| Proposal Number | Proposal Description | Board Recommendation | Vote Required for Approval | Effect of Abstentions | Effect of Broker Non-Vote | ||||||||||||
| 1 | Election of Directors | FOR each director nominee | Nominees receiving the most “For” votes | None | |||||||||||||
| 2 | Advisory | FOR | “For” votes from a majority of | Against | None | ||||||||||||
| 3 | Advisory Vote on Frequency of Future Advisory Votes on Compensation of Our Named Executive Officers | ONE YEAR | Frequency period receiving the most votes | No effect | None | ||||||||||||
| 4 | Ratification of | FOR | “For” votes from a majority of | Against | |||||||||||||
What is the quorum requirement?
A quorum of stockholders is necessary to hold a valid meeting. A quorum will be present if stockholders holding a majority of the outstanding shares entitled to vote are present at the meeting in person via live webcast or represented by proxy. On the record date,Record Date, there were 29,219,1858,116,488 shares outstanding and entitled to vote.Thus, the holders of 14,609,5934,058,245 shares must be present in personat the meeting or represented by proxy at the meeting to have a quorum.
Your shares will be counted toward the quorum only if you submit a valid proxy (or one is submitted on your behalf by your broker, bank or other nominee) or if you vote in person via live webcast at the meeting. Abstentions and broker non-votes will be counted towards the quorum requirement. If there is no quorum, the holders of a majority of shares presentrepresented at the meeting in person or represented by proxy may adjourn the meeting to another date.
5
How can I find out the results of the voting at the annual meeting?
Preliminary voting results will be announced at the Annual Meeting. In addition, final voting results will be published in a current report on Form 8-K that we expect to file within four business days after the Annual Meeting. If final voting results are not available to us in time to file a Form 8-K within four business days after the Annual Meeting, we intend to file a Form 8-K to publish preliminary results and, within four business days after the final results are known to us, file an additional Form 8-K to publish the final results.
PROPOSAL 1
ELECTION OF DIRECTORS
Our Board presently has eightseven members. There are threetwo directors in the class whose term of office expires in 2017,2024, each of whom has been nominated for re-election. Charles J. Link, Jr., M.D.Chad A. Johnson currently serves on our boardBoard and was previously elected by the stockholders in 2014. Thomas A. Raffin2021. Lota S. Zoth currently serves on our boardBoard and was previously elected by the stockholders in 2014. Paolo Pucci currently serves on our board and was elected to fill a vacancy on our Board on November 12, 2015.2021. If elected at the Annual Meeting, each nominee would serve until the 20202027 Annual Meeting of Stockholders and until hissuch person's successor has been duly elected and qualified, or, if sooner, until the director’s death, resignation or removal. It is our policy to encourage directors and nominees for director to attend the Annual Meeting. Seven of the eight directors continuing their service as members of our Board after the 2016 Annual Meeting of Stockholders attended the meeting.
Directors are elected by a plurality of the votes of the holders of shares present in person at the Annual Meeting or represented by proxy and entitled to vote on the election of directors. The three nominees receivingIf a choice is specified on the highest number of affirmative votesproxy card by a stockholder, their shares will be elected.voted as specified. If a choice is not specified on the proxy card, and authority to do so is not withheld, the shares will be voted “FOR” the election of nominees named below. Shares represented by executed proxies will be voted, if authority to do so is not withheld, for the election of the nominees named below. If any nominee becomes unavailable for election as a result of an unexpected occurrence, shares that would have been voted for that nominee will instead be voted for the election of a substitute nominee proposed by us. Each person nominated for election has agreed to serve if elected. Our management has no reason to believe that any nominee will be unable to serve.
Class IIIII Director Nominees
Below is a brief biography of each nominee and each director whose term will continue after the Annual Meeting, including the ages of each nominee and director as of April 6, 2016.1, 2024. Each individual listed below is nominated for election for a three-year term expiring at the 20202027 Annual Meeting.
| Name of Nominee | Age | Position Held with Company | Committees | Director Since |
| Charles J. Link, Jr., M.D. | 57 | Chairman of the Board and Chief Executive and Scientific Officer | None | 2011 |
| Paolo Pucci | 55 | Director | Nominating and Corporate Governance Committee and Audit Committee | 2015 |
| Thomas A. Raffin | 70 | Director | Nominating and Corporate Governance Committee and Compensation Committee | 2011 |
| Name of Nominee | Age | Position Held with Company | Committees | Director Since | ||||||||||
| Chad A. Johnson | 45 | Director | Audit Nominating and Corporate Governance | 2018 | ||||||||||
| Lota S. Zoth | 64 | Director | Audit Compensation | 2012 | ||||||||||
Chad A. Johnson, J.D.,age 57, founded NewLink Genetics Corporation in 1999 and has served as Chairman of our Board and our Chief Scientific Officer since inception in 1999. He served as President from 2001 to 2009 and has served as Chief Executive Officer since 2003. From 1995 to 2013, Dr. Link was a practicing oncologist at the Medical Oncology and Hematology Associates of Iowa. From 1995 to 2003, Dr. Link served as the Director of the John Stoddard Cancer Research Institute, which he co-founded. Dr. Link served as a Medical Oncology Clinical Fellow at the National Cancer Institute and National Institutes of Health from 1988 to 1991. Dr. Link attended the U.S. Air Force Academy from 1977 to 1980. Dr. Link holds a B.A. from Stanford University, an M.D. from Stanford University School of Medicine and is certified in Internal Medicine by the American Board of Internal Medicine and has previously been certified in Medical Oncology.
Our Board believes that Mr. Pucci’s extensive drug development track record and business practice in large multinational as well as emergingJohnson’s career at major biotechnology companies, providesservice as a public company officer and experience overseeing various legal matters provide him with the experiencebackground necessary for him to serve as a member of our AuditBoard, our Compensation Committee and the Chair of our Nominating and Corporate Governance Committee.
7
Lota S. Zoth, CPA, age 64, has served as a member of the Board and Chair of the Audit Committee since November 2012. Ms. Zoth currently serves on the Board of Directors of 89Bio Inc., enGene Holdings Inc. and Inovio Pharmaceuticals, Inc. She also previously served on the Board of Directors for nonprofit Aeras from 2011 to 2018, Circassia Pharmaceuticals, PLC from 2015 to 2019, Hyperion Therapeutics, Inc. from 2008 to May 2015, Ikaria, Inc. from 2008 to 2014, Orexigen Therapeutics, Inc. from 2012 to 2019, Spark Therapeutics, Inc. from 2018 to 2019 and Zymeworks, Inc. from 2017 to 2023. Prior to her board service, Ms. Zoth served as Chief Financial Officer of MedImmune, Inc. from 2004 through 2007, and as its Corporate Controller from 2002 to 2004. Prior to that, Ms. Zoth was a financial executive at several companies, including Sodexho Marriott Services, Inc., PSINet Inc., Marriott International, Inc. and PepsiCo, Inc. Ms. Zoth began her career as an auditor at Ernst & Young, LLP. Ms. Zoth received a BBA in accounting, summa cum laude, from Texas Tech University.
Our Board believes that Ms. Zoth’s experience with us, as a director since 2012 and as the current chair of our Audit Committee of our Board and a member of our Compensation Committee of our Board, brings continuity to our Board. In addition, our Nominating and Corporate Governance Committee believes that Ms. Zoth’s extensive financial background and experience provides important experience in corporate finance, corporate management, and investor relations and provides the background necessary for her to serve as a member of our Audit Committee and our Compensation Committee.
OUR BOARD OF DIRECTORS RECOMMENDS A VOTE IN FAVOR OF EACH DIRECTOR NOMINEE.
8
Class I Directors Continuing in Office Until the 2025 Annual Meeting of Stockholders
An van Es-Johansson, M.D. age 64, has served as a member of our Board since February 2021. Dr. van Es-Johansson currently serves as a senior advisor for AlzeCure Pharma, a Swedish pharmaceutical company with a primary focus on Alzheimer's disease. Dr. van Es-Johansson was previously the Chief Medical Officer and Head of Development for AlzeCare Pharma from 2018 to 2021. From 2005 to 2018, Dr. van Es-Johansson served in a range of executive roles of increasing responsibility at Sobi, an international rare disease company headquartered in Stockholm, Sweden. Dr. van Es-Johansson also served as a member of the Scientific Advisory board for Uppsala Bio from 2004 to 2016 and currently serves on the Board of Directors at Medivir AB, Savara Inc., PLUS Therapeutics and Agendia BV. Dr. van Es-Johansson received an M.D. from Erasmus University, Rotterdam, The Netherlands.
Our Board believes that Dr. van Es-Johansson's vast experience in the pharmaceutical industry with a focus on growth hormone disorders and other rare diseases provides immense value to our Board.
Kevin Lalande, age 51, has served on our Board since the Merger and served as a member of the Private Lumos Board from 2014 through the Merger. Mr. Lalande is a Co-Founder and Managing Director of Santé Ventures, a healthcare and life science venture capital firm founded in 2006 which currently manages $380 million across three funds with 30 portfolio company investments. Mr. Lalande is also the Founder and Chief Investment Officer of Santé Capital, a systematic machine learning hedge fund that began trading capital in 2015 after three years of research and development. Mr. Lalande conceived the investment strategy, designed the original MindRank algorithms, and assembled a seasoned team to help drive this related line of business. Before Santé Ventures and Santé Capital, Mr. Lalande spent seven years as an investment professional with Austin Ventures, a prominent venture capital firm with $4.0 billion under management. Prior to Austin Ventures, he was a management consultant with McKinsey & Company. Before McKinsey, he founded, built and sold three internet-based companies in the 1990s. Mr. Lalande received a B.S. in electrical and computer engineering with honors in 1996 from Brigham Young University and an MBA with highest distinction from the Harvard Business School in 2001, where he was both a Baker Scholar and a Siebel Scholar.
Our Board believes that Mr. Lalande’s extensive experience as an investor and board member in pharmaceutical and life sciences companies and his knowledge gained from service on such boards qualify him to be a member of our Board.
Joseph S. McCracken, age 71, has served as a member of our Board since March 2020. Dr. McCracken currently advises biopharmaceutical companies on the design and implementation of corporate strategy and business development initiatives. Dr. McCracken also serves on the boards of Kindred Biosciences, Inc. (NASDAQ: KIN), Savara Inc. (NASDAQ: SVRA) and Modalis Therapeutics, Inc. (TSE: 4883.T), as well as the boards of privately held Regimmune Inc. From July 2011 to September 2013, Dr. McCracken was Vice President and Global Head of Business Development & Licensing for Roche Pharma, a research-focused healthcare company, where he was responsible for Roche Pharma’s global in-licensing and out-licensing activities. From October 2009 until July 2011, he was General Manager, Roche Pharma Japan & Asia Regional Head, Roche Partnering. Prior to joining Roche Pharma, Dr. McCracken held the position of Vice President, Business Development at Genentech for more than nine years, and previously held similar positions at Aventis Pharma and Rhone-Poulenc Rorer. Dr. McCracken holds a B.S. in microbiology, a Master of Science in pharmacology and a Doctorate of Veterinary Medicine from The Ohio State University.
Our Board believes that Dr. McCracken’s extensive experience in the biotechnology and pharmaceutical industries qualifies him to serve on our Board.
9
Class II Directors Continuing in Office Until the 2026 Annual Meeting of Stockholders
Richard J. Hawkins, age 75, has served as Chief Executive Officer and as a member of our Board since the Merger and served as a member of the Private Lumos Board from 2011 through the Merger. In addition, Mr. Hawkins currently serves on the board of directors of several life sciences companies, including Plus Therapeutics, Inc. (Nasdaq: PSTV) and Savara Inc. (Nasdaq: SVRA), and previously served on the board of directors of SciClone Pharmaceuticals, Inc. until its acquisition in October 2017. Mr. Hawkins also served as our President from the Merger through July 2021. From 2000 to 2010, Mr. Hawkins, founded and advised numerous pharmaceutical companies including Sensus, where he served as co-founder and Chair until it was sold to Pfizer. From 1981 to 2000, Mr. Hawkins was founder, President and CEO of Pharmaco. The company later merged with PPD of Wilmington, NC to form PPD Pharmaco, one of the largest clinical contract research organizations in the world. Mr. Hawkins received his B.S. degree in biology from Ohio University.
Our Board believes that Mr. Hawkins’s experience in the pharmaceutical and life sciences industries as well as his broad management experience qualify him to serve on our Board.
Thomas A. Raffin, M.D., age 70,77, has served as a member of ourthe Board since 1999 and is currently ourhas been the Board’s Lead Independent Director.Director since October 2010. Dr. Raffin has spent 30 years on the faculty at Stanford University School of Medicine, where he is the Colleen and Robert Haas Professor Emeritus of Medicine and Biomedical Ethics. Over the past two decades, Dr. Raffin has worked extensively in the healthcare and medical device business sectors and was an advisor to Cell Therapeutics Inc. from 1993 to 1997, Broncus Technologies from 1997 to 2004, iMedica from 1998 to 2002, and Inhale Technologies from 1998 to 2001. He co-founded Rigel Pharmaceuticals, a publicly traded company (Nasdaq: RIGL), in 1996. In 2001, he co-founded Telegraph Hill Partners, a San Francisco life sciences private equity firm as a General Partner. Dr. Raffin has been a director of the following Telegraph Hill Partners private portfolio companies: AngioScore, Inc., Confirma, Inc., Freedom Innovations, LDR Holding Corporation, MagstimPneumRx, Inc., Akoya BioSciences, Inc. and PneumRx; and he has worked closely with Estech and Vidacare.InvisALERT Solutions. Dr. Raffin received a B.A. from Stanford University and an M.D. from Stanford University School of Medicine and did his medical residency at the Peter Bent Brigham Hospital (now Brigham and Women'sWomen’s Hospital) in Boston, MA.
Our Board believes that Dr. Raffin’s extensive medical and business background and experience provides important experience in business operations and medical technology and provides the background necessary for him to serve as a member of our Compensation Committee and our Nominating and Corporate Governance Committee.
10
INFORMATION REGARDING OUR BOARD OF DIRECTORS AND CORPORATE GOVERNANCE
Independence of our Board of Directors
In determining independence, our Board considers the definition of “independent” set forth in the listing standards of the NASDAQ Stock Market, or NASDAQ, as well as other factors that contribute to effective oversight and decision-making by our Board. Our independence standards are set forth in our Corporate Governance Guidelines on our website at www.newlinkgenetics.comwww.lumos-pharma.com in the “Investors & Media - Corporate Governance - Corporate Governance Guidelines”Governance” section. As required under the NASDAQ listing standards, a majority of the members of a listed company’s board of directors must qualify as “independent,” as affirmatively determined by our Board. Our Board consults with our counsel to ensure that our Board’s determinations are consistent with relevant securities and other laws and regulations regarding the definition of “independent,” including those set forth in pertinent listing standards of NASDAQ, as in effect from time to time.
Consistent with these considerations, after review of all relevant identified transactions or relationships between each director, or any of his or her family members and our Company, its senior management and its independent auditors, our Board has affirmatively determined that the following six directors who served on our Board in 2016 and continue to serve (including nominees for election at the Annual Meeting, Mr. Pucci and Dr. Raffin) are independent directors within the meaning of the applicable NASDAQ listing standards: Dr. van Es-Johansson, Mr. Edick,Johnson, Mr. Saluri, Mr. Talarico, Ms. Zoth,Lalande, Dr. McCracken, Dr. Raffin and Mr. Pucci.Ms. Zoth. In making this determination,its independence assessments, our Board found that none of these directors or nominees for director had a material or other disqualifying relationship with our Company.
There are no family relationships betweenamong any of our directors, director nominees and executive officers.
Board Leadership
Our Board is currently chaired by theour Chief Executive Officer, of our Company, Dr. Charles J. Link, Jr.Mr. Hawkins. Our Board has appointed Dr. Raffin as Lead Independent Director.
Our Board appointed Dr. Raffin as the Lead Independent Director to help reinforce the independence of our Board as a whole. The position of Lead Independent Director has been structured to serve as an effective balance to a combined Chief Executive Officer/Chairman: theOfficer and Chair. The Lead Independent Director is empowered, among other duties and responsibilities, to develop, together with the Chief Executive Officer, the agenda for meetings of our Board, to develop, together with committee chairs, the agendas for meetings of committees, to preside over Board meetings in the absence of the officers and to oversee our Board’s annual evaluation of the Chief Executive Officer’s performance.
11
Role of Our Board of Directors on Risk Oversight
One of our Board’s key functions is informed oversight of our risk management process. Our Board does not have a standing risk management committee, but rather administers this oversight function directly through our Board as a whole, as well as through various Board standing committees that address risks inherent in their respective areas of oversight. In particular, while our Board is responsible for monitoring and assessing strategic risk exposure, our Audit Committee has the responsibility to consider and discuss the major financial risk exposures and the steps management has taken to monitor and control these exposures, including guidelines and policies to govern the process by which risk assessment and management is undertaken. Our Audit Committee also monitors compliance with legal and regulatory requirements with respect to SEC regulations, and NASDAQ listing standards, pharmaceutical industry regulations and corporate risk management, in addition to oversight of the performance of our accounting and financial reporting processes. Our Nominating and Corporate Governance Committee monitors the effectiveness of the corporate governance guidelines, including whether they are successful in preventing illegal or improper liability-creating conduct.conduct, and is responsible for overseeing our cybersecurity risk management processes. Our Compensation Committee assesses and monitors whether any compensation policies and programs have the potential to encourage excessive risk-taking. The entire Board and its committees address risk management issues from time-to-time and meet at least annually with the employees responsible for risk management in the committees’ respective areas of oversight. Both our Board as a whole and the various standing committees receive periodic reports from the employees responsible for risk management, as well as incidental reports as matters may arise. It is the responsibility of the committee chairs to report findings regarding material risk exposures to our Board as quickly as possible.
Meeting Attendance
Our Board met seven times during the last fiscal year.year ended December 31, 2023. Our Audit Committee met four times during the 2023 fiscal year, our Compensation Committee met five times during the 2016 fiscal year, our Compensation Committee met eight times during the 20162023 fiscal year, and our Nominating and Corporate Governance Committee met four times. Eachtimes during the 2023 fiscal year. None of our incumbent directordirectors attended 100%fewer than 75% of the aggregate number of meetings of ourthe Board and committee meetings of the committees on which he or she served, that were held during the portion of the last fiscal year for which he or she was a member.
It is our policy to encourage directors and nominees for director or committee member withto attend the exception that Mr. Talarico was absent from the BoardAnnual Meeting. Each of Directors meetings that took place on May 6 and July 15, 2016.our directors attended our virtual 2023 Annual Meeting of Stockholders.
Committees of our Board of Directors
Audit Committee, Compensation Committee and Nominating and Corporate Governance Committee:
| Name | Audit | Compensation | Nominating and Corporate Governance | ||||||||
| X | |||||||||||
| X | Chair | ||||||||||
| Kevin Lalande | X | ||||||||||
| Joseph McCracken | X | ||||||||||
| Thomas A. Raffin, M.D. | Chair | X | |||||||||
| Chair | X | ||||||||||
Each of the committees has authority to engage legal counsel or other experts or consultants, as it deems appropriate to carry out its responsibilities. Our Board has determined that, except as specifically described below, each current member of each committee meets the applicable NASDAQ rules and regulations regarding “independence” and that each member is free of any relationship that would impair his or her individual exercise of independent judgment with regard to the Company.
12
Board Diversity
We value diverse perspectives and believe different points of view brought through diverse representation lead to better business performance, decision making and understanding. Our Board takes a multi-dimensional approach to diversity. In addition to industry expertise and professional experience, our Board values representation that reflects diversity in other important categories including gender, race/ethnicity and sexual orientation.
The matrix below summarizes our current board composition:
| Board Diversity Matrix (As of March 31, 2024) | ||||||||
| Board Size: | ||||||||
| Total Number of Directors | 7 | |||||||
| Gender | Male | Female | ||||||
| Number of directors based on gender identity | 5 | 2 | ||||||
| Number of directors who identify in any of the categories below: | ||||||||
| White | 5 | 2 | ||||||
| LGBTQ+ | 1 | — | ||||||
Below is a description of each committee of our Board.
Audit Committee
Our Audit Committee was established by our Board in accordance with Section 3(a)(58)(A) of the Securities Exchange Act of 1934, as amended, or the Exchange Act to oversee our corporate accounting and financial reporting processes and audits
of itsour financial statements. For this purpose, our Audit Committee performs several functions. Our Audit Committee evaluates the performance of and assesses the qualifications of the independent auditors; determines and approves the engagement of the independent auditors; determines whether to retain or terminate the existing independent auditors or to appoint and engage new independent auditors; reviews and approves the retention of the independent auditors to perform any proposed permissible non-audit services; monitors the rotation of partners of the independent auditors on our audit engagement team as required by law; confers with management and the independent auditors regarding the effectiveness of internal controls over financial reporting; establishes procedures, as required under applicable law, for the receipt, retention and treatment of complaints received by the Company regarding accounting, internal accounting controls or auditing matters and the confidential and anonymous submission by employees of concerns regarding questionable accounting or auditing matters;matters, oversees corporate risk management of the company as a whole; and meets to review our annual audited financial statements and quarterly financial statements with management and the independent auditor, including a review of our disclosures in our Annual Report on Form 10-K under the heading “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
Our Audit Committee is currently comprised of fourthree directors: Ms. Zoth, Mr. Edick, Mr. Talarico,Johnson and Mr. Pucci.Dr. McCracken. Our Board has adopted a written Audit Committee charter that is available to stockholders on our website at www.newlinkgenetics.comwww.lumos-pharma.com in the “Investors & Media - Corporate Governance” section.
Our Board reviews the NASDAQ listing standards definition of independence for Audit Committee members on an annual basis and has determined that each current member of our Audit Committee meets the independence requirement (as independence is currently defined in Rule 5605(c)(2)(A)(i) and (ii) of the NASDAQ listing standards).
Our Board has also determined that Ms. Zoth qualifies as an “audit committee financial expert,” as defined in applicable SEC rules. Our Board made a qualitative assessment of Ms. Zoth’s level of knowledge and experience based on a number of factors, including her formal education and her years of experience.
Compensation Committee
The Compensation Committee of our Board is currently comprised of fourthree directors: Dr. Raffin, Mr. Saluri, Mr. TalaricoLalande, and Ms. Zoth. All current members of our Compensation Committee are independent (as independence is currently defined in Rule 5605(d)(2) of the NASDAQ listing standards). Additionally, all current members of our Compensation Committee are "outside directors" for 162(m) purposes and non-employee directors under Rule 16b-3 of the Exchange Act. Our Board has adopted a written Compensation Committee charter
13
that is available to stockholders on our website at www.newlinkgenetics.comwww.lumos-pharma.com in the “Investors & Media - Corporate Governance” section.
The purpose of our Compensation Committee is to discharge the responsibilities of our Board to oversee our compensation policies, plans and programs and to review and determine the compensation to be paid to our directors, executive officers and other senior management. The scope of authority and specific responsibilities of our Compensation Committee include:
•determining the compensation and other terms of employment of our executive officers and reviewing and approving corporate performance goals and objectives relevant to such compensation;
•evaluating and recommending to our Board the compensation plans and programs advisable for the Company, and evaluating and recommending the modification or termination of existing plans and programs;
•reviewing and approving the terms of any employment agreements, severance arrangements, change in control protections and any other compensatory arrangements for our executive officers;
•selecting, retaining and terminating compensation consultants to assist in its evaluation of executive and director compensation, including the sole authority to approve the consultant’s reasonable fees and other retention terms; and
•reviewing and recommending to our Board the type and amount of compensation to be paid or awarded to members of our Board.
Annually, our Compensation Committee Interlockshas considered the following six factors, as set forth by the SEC and Insider Participation
interest that would adversely affect Setren & Associates' independence.
Nominating and Corporate Governance Committee
Our Nominating and Corporate Governance Committee of our Board is responsible for overseeing our corporate governance functions on behalf of our Board, making recommendations to our Board regarding corporate governance issues, identifying, reviewing and evaluating candidates to serve as directors of the Company consistent with criteria approved by our Board, reviewing and evaluating incumbent directors, recommending to our Board for selection candidates for election to our Board and making other recommendations to our Board regarding affairs relating to the directors of the Company, including director compensation.
Our Nominating and Corporate Governance Committee is currently comprised of fourthree directors: Mr. Pucci,Johnson, Dr. Raffin, Mr. Salurivan Es-Johansson and Mr. Edick.Dr. Raffin. All current members of our Nominating and Corporate Governance Committee are independent (as independence is currently defined in Rule 5605(a)(2) of the NASDAQ listing standards). Our Board has adopted a written Nominating and Corporate Governance Committee charter that is available to stockholders on our website at www.newlinkgenetics.comwww.lumos-pharma.com in the “Investors & Media - Corporate Governance” section.
Our Nominating and Corporate Governance Committee believes that candidates for director should have certain minimum qualifications, including the ability to read and understand basic financial statements, being over 21 years of age and having the highest personal integrity and ethics. Our Nominating and Corporate Governance Committee also considers whether the candidate possesses the following factors among others: relevant expertise upon which to base advice and guidance to management, sufficient time to devote to the affairs of the Company, demonstrated excellence in his or her field, the ability to exercise sound business judgment and the commitment to rigorously represent the long-term interests of our stockholders. Candidates for director nominees are reviewed in the context of the current composition of our Board, the operating requirements of the Company and the long-term interests of stockholders. In conducting this assessment, our Nominating and Corporate Governance Committee typically considers diversity, age, skills and such other factors as it deems appropriate given the current needs of our Board and the Company to maintain a balance of knowledge, experience and capability. Our
14
Nominating and Corporate Governance Committee does not have a policy regarding how it considers diversity in selecting candidates.
In the case of incumbent directors whose terms of office are set to expire, our Nominating and Corporate Governance Committee reviews these directors’ overall service to the Company during their terms, including the number of meetings attended, level of participation, quality of performance and any relationships and transactions that might impair the directors’ independence. Our Nominating and Corporate Governance Committee also takes into account the results of our Board’s self-evaluation, conducted annually on a group and individual basis. In the case of new director candidates, our Nominating and Corporate Governance Committee also determines whether the nominee is independent for NASDAQ purposes, which determination is based upon applicable NASDAQ listing standards, applicable SEC rules and regulations and the advice of counsel, if necessary. Our Nominating and Corporate Governance Committee then uses its network of contacts to compile a list of potential candidates, but may also engage, if it deems appropriate, a professional search firm. Our Nominating and Corporate Governance Committee conducts any appropriate and necessary inquiries into the backgrounds and qualifications of possible candidates after considering
the function and needs of our Board. Our Nominating and Corporate Governance Committee meets to discuss and consider the candidates’ qualifications and then selects a nominee for recommendation to our Board by majority vote. During 2015, our Nominating and Corporate Governance Committee retained and paid a search firm to assist in the identification and evaluation of candidates for director.
In identifying potential candidates for Board membership, our Nominating and Corporate Governance Committee relies on suggestions and recommendations from our Board, stockholders, management and others. Our Nominating and Corporate Governance Committee will consider director candidates recommended by stockholders.stockholders that are properly and timely submitted by stockholders in accordance with our policies and Bylaws. Our Nominating and Corporate Governance Committee does not intend to alter the manner in which it evaluates candidates, including the minimum criteria set forth above based on whether or not the candidate was recommended by a stockholder.
Code of Business Conduct and Ethics
The Company has adopted the NewLink Genetics Corporationits Code of Business Conduct and Ethics that applies to all officers, directors and employees. The Code of Business Conduct and Ethics is available on our website at www.newlinkgenetics.comwww.lumos-pharma.com in the “Investors & Media - Corporate Governance” section. The Company amended the code of ethics in October 2015 and anyAny future amendments or waivers to our code of ethics will be promptly disclosed on itsour website and as required by applicable laws, rules and regulations of the SEC and NASDAQ.
Corporate Governance Guidelines
Our Board adopted Corporate Governance Guidelines to assure that our Board will have the necessary authority and practices in place to review and evaluate our business operations as needed and to make decisions that are independent of our management. The guidelines are also intended to align the interests of directors and management with those of our stockholders. The Corporate Governance Guidelines set forth the practices our Board intends to follow with respect to board composition and selection, board meetings and involvement of senior management, Chief Executive Officer performance evaluation and succession planning and board committees and compensation. The Corporate Governance Guidelines, as well as the charters for each committee of our Board, may be viewed at www.newlinkgenetics.comwww.lumos-pharma.com in the “Investors & Media - Corporate Governance” section.
Executive Sessions of Independent Directors
To encourage and enhance communication among independent directors, and as required under applicable Nasdaq rules, our Corporate Governance Guidelines provide that the independent directors meet in executive sessions without management directors or management present on a periodic basis, no less than four times per year. These executive sessions are chaired by Dr. Raffin as Lead Independent Director.
15
PROPOSAL 2
ADVISORY VOTE ON COMPENSATION OF OUR NAMED EXECUTIVE COMPENSATIONOFFICERS
At the 20122018 Annual Meeting of Stockholders, theour stockholders indicated their preference that the Company solicit a non-binding advisory vote on the compensation of the named executive officers, commonly referred to as a “say-on-pay vote,” every year. Our Board has adopted a policy that is consistent with that preference.
This vote is being provided pursuant to sectionSection 14A of the Securities Exchange Act. It is not intended to address any specific item of compensation, but rather the overall compensation of our named executive officers and the philosophy, policies and practices described in this proxy statement. The compensation of our named executive officers subject to the vote is disclosed in the Compensation Discussion and Analysis, the compensation tables and the related narrative disclosure contained in this proxy statement. As discussed in those disclosures, the Company believes that its compensation policies and decisions are consistent with our strategic compensation and retention needs. Further, our compensation policies and decisions are designed to align itsour executive officers’ compensation with our business objectives and the interests of its stockholders, to incentivize and reward itsour executive officers for our success and to promote teamwork within our executive management team. Compensation of our named executive officers is designed to enable the Company to attract and retain talented and experienced executives to lead the Company successfully in a competitive environment.
Accordingly, our Board is asking the stockholders to indicate their support for the compensation of our named executive officers as described in this proxy statement by casting a non-binding advisory vote “FOR” the following resolution:
“RESOLVED, that the compensation paid to our named executive officers, as disclosed pursuant to Item 402 of Regulation S-K, including the Compensation Discussion and Analysis, compensation tables and narrative discussion, is hereby APPROVED.”
Because the vote is advisory, it is not binding on our Board or our Company. Nevertheless, the views expressed by the stockholders, whether through this vote or otherwise, are important to management and our Board and, accordingly, our Board and our Compensation Committee intend to consider the results of this vote in making determinations in the future regarding executive compensation arrangements.
Advisory approval of this proposal requires the affirmative vote of the holders of a majority of the shares present in person or represented by proxy at the Annual Meeting and entitled to vote generally on the matter at the Annual Meeting.subject matter. Abstentions will be counted toward the tabulation of votes on proposals presented to the stockholders and will have the same effect as negative votes. Broker non-votes are counted toward a quorum but are not counted for any purpose in determining whether this matter has been approved.
Unless our Board decides to modify its policy regarding the frequency of soliciting advisory votes on the compensation of our named executive officers, the next scheduled say-on-pay vote will be at the 20182025 Annual Meeting of Stockholders.
OUR BOARD OF DIRECTORS RECOMMENDS A VOTE IN FAVOR OF PROPOSAL 2.
16
PROPOSAL 3
ADVISORY VOTE ON THE FREQUENCY OF FUTURE ADVISORY VOTES ON THE COMPENSATION OF OUR NAMED EXECUTIVE OFFICERS
The Dodd-Frank Act enables our stockholders to indicate how frequently they believe we should seek a non-binding advisory vote from our stockholders on the compensation of our named executive officers, i.e., how frequently to request future “Say-on-Pay” votes from stockholders. We are accordingly seeking a non-binding, advisory vote from stockholders as to the frequency with which our stockholders should have an opportunity to provide an advisory approval of our named executive officer compensation. We are providing our stockholders with the choice of selecting a frequency of one year, two years or three years, or abstaining from this advisory vote. The alternative of every one year, two years, or three years that receives the highest number of votes cast by stockholders in person or by proxy at this meeting will be considered to be the preferred frequency that has been recommended by our stockholders.
The vote on this proposal is advisory; therefore, it is not binding on the Company, our Board or our Compensation Committee. We may determine in the future that it is in the best interests of the Company and our stockholders to hold Say-on-Pay votes more or less frequently than the frequency indicated by stockholders in voting on this proposal or as currently recommended by our Board. However, we plan to consider the results of the vote on this proposal in determining the frequency of our Say-on-Pay votes because we value the opinions of our stockholders.
Currently, we believe that it is in the best interests of the Company and our stockholders to hold a Say-on-Pay vote every year, and this is the frequency recommended by our Board. We believe this frequency will enable our stockholders to vote, on a non-binding, advisory basis, on our most recent executive compensation practices and decisions as presented in our annual proxy statements, which will lead to greater transparency and more meaningful and timely communication between us and our stockholders regarding the compensation of our named executive officers.
Accordingly, we recommend that our stockholders indicate a preferred voting frequency period of “One Year” in response to the following non-binding advisory resolution:
“RESOLVED, that the alternative of every one year, two years, or three years that receives the highest number of votes cast by stockholders in person or by proxy at this meeting will be considered to be the preferred frequency with which the Company is to hold an advisory vote on the compensation of the Company’s named executive officers.”
OUR BOARD OF DIRECTORS RECOMMENDS THAT STOCKHOLDERS VOTE FOR THE OPTION OF ONE YEAR AS THE PREFERRED FREQUENCY PERIOD.
17
PROPOSAL 4
RATIFICATION OF SELECTION OF KPMG LLP AS INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Our Audit Committee has selected KPMG LLP as our independent registered public accounting firm for the fiscal year ending December 31, 20172024 and has further directed that management submit the selection of independent registered public accounting firm for ratification by the stockholders at the Annual Meeting. KPMG LLP has audited our financial statements since inception in 1999. From 2015 through the Merger, KPMG LLP audited Private Lumos' financial statements. Representatives of KPMG LLP are expected to be presentavailable at the Annual Meeting. They will have an opportunity to make a statement if they so desire and will be available to respond to appropriate questions.
Neither our Bylaws nor other governing documents or law require stockholder ratification of the selection of KPMG LLP as our independent registered public accounting firm. However, our Audit Committee is submitting the selection of KPMG LLP to the stockholders for ratification as a matter of good corporate practice. If the stockholders fail to ratify the selection, our Audit Committee will reconsider whether or not to retain that firm. Even if the selection is ratified, our Audit Committee in its discretion may direct the appointment of different independent auditors at any time during the year if they determine that such a change would be in the best interests of our Company and our stockholders.
The affirmative vote of the holders of a majority of the shares present in person or represented by proxy at the Annual Meeting and entitled to vote generally on the subject matterat the Annual Meeting will be required to ratify the selection of KPMG LLP. Abstentions will be counted toward the tabulation of votes on proposals presented to the stockholders and will have the same effect as negative votes. Broker non-votes are counted towards a quorum but are not counted for any purpose in determining whether this matter has been approved.
Principal Accountant Services and Fees
The following table representspresents the aggregate fees billed to the Company for the fiscal years ended December 31, 20162023 and December 31, 2015,2022, by KPMG LLP, our principal accountant. All tax fees described below were pre-approved by our Audit Committee.
| Year Ended | ||||||||||||||||||
| 2016 | 2015 | Year Ended December 31, | ||||||||||||||||
| 2023 | 2022 | |||||||||||||||||
Audit Fees (1) | $507,750 | $577,300 | Audit Fees (1) | $480,000 | $475,000 | |||||||||||||
| Audit-related Fees | — | — | Audit-related Fees | 0 | ||||||||||||||
Tax Fees (2) | $140,607 | $202,048 | Tax Fees (2) | $100,000 | $122,000 | |||||||||||||
| All Other Fees | — | — | All Other Fees | 0 | ||||||||||||||
| Total Fees | $648,357 | $779,348 | Total Fees | $580,000 | $597,000 | |||||||||||||
(1)Represents fees for the audit of our annual financial statements, and of our internal control over financial reporting, review of our quarterly financial statements included in our Forms 10-Q, accounting consultations and the issuance of consents and comfort letters.
(2)Consists of fees for tax services provided to the Company, including tax planning and compliance services and the review of certain tax returns.returns.
Pre-Approval Policies and Procedures
Our Audit Committee has adopted a policy and procedures for the pre-approval of audit services, audit-related services and tax services rendered by our independent registered public accounting firm. The policy generally pre-approves specified services in the defined categories of audit services, audit-related services, and tax services up to specified amounts. Pre-approval may also be given as part of our Audit Committee’s approval of the scope of the engagement of the independent registered public accounting firm or on an individual explicit case-by-case basis before the independent registered public accounting firm is engaged to provide each service. The pre-approval of services has been delegated to the ChairpersonChair of our Audit Committee, but the decision must be reported to the full Audit Committee at its next scheduled meeting.
18
In connection with the audit of the 20162023 financial statements, our Audit Committee entered into an engagement agreement with KPMG LLP whichthat sets forth the terms by which KPMG LLP was to perform audit services for the Company.
Report of Our Audit Committee (1)
Our Audit Committee has reviewed and discussed the audited financial statements for the fiscal year ended December 31, 20162023 with management of the Company. Our Audit Committee has discussed with the independent registered public accounting firm the matters required to be discussed by Auditing Standard No. 16,1301, Communications with Audit Committee, as adopted by the Public Company Accounting Oversight Board, or PCAOB. Our Audit Committee has also received the written disclosures and the letter from the independent registered public accounting firm required by applicable requirements of the PCAOB regarding the independent accountants’ communications with our Audit Committee concerning independence, and has discussed with the independent registered public accounting firm the accounting firm’s independence.
Based on the foregoing, our Audit Committee has recommended to our Board of Directors that the audited financial statements be included in our Annual Report on Form 10-K for the fiscal year ended December 31, 2016.2023, filed March 7, 2024.
AUDIT COMMITTEE
Ms. Lota Zoth (Chair)
Joseph S. McCracken
(1)The material in this Audit Committee report is not “soliciting material,” is not deemed “filed” with the Commission and is not to be incorporated by reference in any filing of the Company under the Securities Act or the Exchange Act, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
OUR BOARD OF DIRECTORS RECOMMENDS A VOTE IN FAVOR OF PROPOSAL 3.4.
19
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information regarding the ownership of our common stock as of March 15, 2017,1, 2024, except as set forth below, by: (i) each current director and nominee for director; (ii) each of the named executive officers specified in the Summary Compensation Table; (iii) all executive officers and directors of the Company as a group; and (iv) all those known by the Company to be beneficial owners of more than 5% of its common stock.
| Names of Beneficial Owner | Shares | Percentage | |||||
| 5% Stockholders | Total | ||||||
Stine Seed Farm, Inc. (1) | 7,077,245 | 24.2 | % | ||||
First Eagle Investment Management, LLC (2) | 2,848,000 | 9.7 | % | ||||
OrbiMed Advisors (3) | 1,991,700 | 6.8 | % | ||||
BlackRock Inc. (4) | 1,825,915 | 6.2 | % | ||||
| Directors and Named Executive Officers | |||||||
Charles J. Link, Jr., M.D. (5) | 2,103,801 | 6.8 | % | ||||
Nicholas N. Vahanian, M.D. (6) | 1,236,631 | 4.1 | % | ||||
John B. Henneman, III (7) | 257,771 | * | |||||
Thomas A. Raffin, M.D. (8) | 211,721 | * | |||||
Carl Langren (9) | 186,702 | * | |||||
Brian Wiley (10) | 165,064 | * | |||||
Ernest J. Talarico, III (11) | 159,683 | * | |||||
Joseph B. Saluri (12) | 85,527 | * | |||||
Paul R. Edick (13) | 65,721 | * | |||||
Lota S. Zoth (14) | 64,531 | * | |||||
Paolo Pucci (15) | 14,021 | * | |||||
| Total for Security Ownership Table | 18,294,033 | 55.0 | % | ||||
All Executive Officers & Directors as a Group (11 persons)(16) | 4,551,173 | 13.7 | % | ||||
20
Name and Address of Beneficial Owner(1) | Shares (#) | Percent (%) | ||||||||||||
| 5% and Greater Stockholders | ||||||||||||||
Richard J. Hawkins(2) | 949,879 | 11.4% | ||||||||||||
Stine Seed Farm, Inc.(3) | 873,081 | 10.8% | ||||||||||||
Kevin Lalande(4) | 743,547 | 9.2% | ||||||||||||
Goldman Sachs Group Inc.(5) | 406,711 | 5.0% | ||||||||||||
| Named Executive Officers and Directors | ||||||||||||||
Richard J. Hawkins(2) | 949,879 | 11.4% | ||||||||||||
Kevin Lalande(4) | 743,547 | 9.2% | ||||||||||||
John McKew, Ph.D.(6) | 206,959 | 2.5% | ||||||||||||
Lori D. Lawley(7) | 72,263 | * | ||||||||||||
Aaron Schuchart(8) | 69,824 | * | ||||||||||||
Bradley J. Powers(9) | 59,143 | * | ||||||||||||
Thomas A. Raffin, M.D.(10) | 34,647 | * | ||||||||||||
Joseph McCracken(11) | 27,746 | * | ||||||||||||
Lota S. Zoth(12) | 22,313 | * | ||||||||||||
Chad A. Johnson(13) | 20,105 | * | ||||||||||||
An van Es-Johansson, M.D.(14) | 12,925 | * | ||||||||||||
All current executive officers and directors as a group (11 persons)(15) | 2,219,351 | 25.4% | ||||||||||||
| * | Indicates beneficial ownership of less than 1% of the outstanding shares of the Company’s common stock. | |||||||||||||
| (1) | Unless otherwise indicated, the address of such individual is Lumos Pharma, Inc., 4200 Marathon Boulevard, Suite 200, Austin, Texas 78756. | |||||||||||||
| (2) | Includes 201,503 shares Mr. Hawkins has the right to acquire through the exercise of stock options and 6,250 RSUs that are scheduled to vest, each within 60 days of March 1, 2024. | |||||||||||||
| (3) | Address is 22555 Laredo Trail, Adel, Iowa 50003. Information presented is based solely upon a Schedule 13D filed with the SEC on October 6, 2017. Harry H. Stine, the CEO of Stine Seed Farm, Inc., may be deemed to beneficially own such shares. | |||||||||||||
| (4) | Consists of the shares held by Sante Health Ventures II, L.P. Mr. Lalande may be deemed to beneficially own such shares. Mr. Lalande, Joe Cunningham, M.D. and Douglas D. French, are managing directors (the “SHV Directors”) of SHV Management Services, LLC (“SHV Management”). SHV Management is the general partner of SHV Management Services, LP, which is the general partner of Santé Health Ventures II, L.P. Each of the SHV Directors, SHV Management, and SHV Management Services, LP disclaims beneficial ownership of these securities except to the extent of its or his pecuniary interest therein. The address for this entity is 201 W 5th Street, Suite 1500, Austin, TX 78701. Information presented is based solely upon a Schedule 13D filed with the SEC on August 30, 2021. Includes 12,025 shares Mr. Lalande has the right to acquire through the exercise of stock options within 60 days of March 1, 2024. | |||||||||||||
| (5) | The securities being reported on by The Goldman Sachs Group, Inc. ("GS Group"), as a parent holding company, are owned, or may be deemed to be beneficially owned, by Goldman Sachs & Co. LLC ("Goldman Sachs"), a broker or dealer registered under Section 15 of the Act and an investment adviser registered under Section 203 of the Investment Advisers Act of 1940. Goldman Sachs is a subsidiary of GS Group. Address is 200 West Street, New York, NY 10282. Information presented is based solely upon a Schedule 13G filed with the SEC on February 9, 2024. | |||||||||||||
| (6) | Includes 201,076 shares Dr. McKew has the right to acquire through the exercise of stock options and 3,250 RSUs that are scheduled to vest, each within 60 days of March 1, 2024. | |||||||||||||
| (7) | Includes 63,819 shares Ms. Lawley has the right to acquire through the exercise of stock options and 1,125 RSUs that are scheduled to vest, each within 60 days of March 1, 2024. | |||||||||||||
| (8) | Includes 64,569 shares Mr. Schuchart has the right to acquire through the exercise of stock options within 60 days of March 1, 2024. | |||||||||||||
| (9) | Includes 54,710 shares Mr. Powers has the right to acquire through the exercise of stock options and 1,125 RSUs that are scheduled to vest, each within 60 days of March 1, 2024. | |||||||||||||
| (10) | Includes 25,241 shares Dr. Raffin has the right to acquire through the exercise of stock options within 60 days of March 1, 2024. | |||||||||||||
| (11) | Includes 12,025 shares Mr. McCracken has the right to acquire through the exercise of stock options within 60 days of March 1, 2024. | |||||||||||||
| (12) | Includes 20,126 shares Ms. Zoth has the right to acquire through the exercise of stock options within 60 days of March 1, 2024. | |||||||||||||
| (13) | Includes 19,029 shares Mr. Johnson has the right to acquire through the exercise of stock options within 60 days of March 1, 2024. | |||||||||||||
| (14) | Includes 12,070 shares Ms. van Es-Johansson has the right to acquire through the exercise of stock options within 60 days of March 1, 2024. | |||||||||||||
| (15) | Includes 686,193 shares issuable pursuant to stock options that the executive officers and directors of the Company have the right to acquire, and 11,750 RSUs owned by the executive officers and directors that vest, within 60 days of March 1, 2024. | |||||||||||||
We maintain our 2019 Equity Incentive Plan, 2010 Non-Employee Directors’ Stock Award Plan and the ESPP, each of which was approved by our security holders, pursuant to which we may grant equity awards to eligible persons. We also maintain the Lumos Plans that were assumed at the closing of the Exchange Act requiresMerger approved by our directors, executive officerssecurity holders.
The following table gives information about equity awards under the applicable foregoing plans as of December 31, 2023:
| Plan Category | Number of Securities to be Issued upon Exercise of Outstanding Options, Warrants and Rights | Weighted-Average Exercise Price of Outstanding Options, Warrants and Rights | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column(a)) | |||||||||||||||||||||||
| Equity compensation plans approved by security holders | 1,399,025 | $ | 8.91 | 753,596 | (1)(2) | |||||||||||||||||||||
| Equity compensation plans not approved by security holders | — | $ | — | — | ||||||||||||||||||||||
| Total | 1,399,025 | 753,596 | ||||||||||||||||||||||||
(1) The 2009 Equity Incentive Plan incorporated an evergreen formula pursuant to which, on each January 1st, the aggregate number of shares reserved for issuance under the plan will increase by a number equal to 4% of the outstanding shares on December 31st of the preceding calendar year, or such lesser amount (or no shares) as determined by our Board. On May 9, 2019, the Company’s stockholders approved a proposal to amend and persons who own more than 10%extend the 2009 Plan (the "2019 Plan") which, among other modifications, included decreasing the automatic annual “evergreen provision” from 4% to 3%, in accordance with which, on January 1 of each year, from 2020 to (and including) 2029, a registered classnumber of our equity securities, to file with the SEC initial reports of ownership and reports of changes in ownershipshares of common stock and other equity securitiesin an amount equal to 3% of the Company. Directors, executive officers and greater than 10% stockholders are required by SEC regulation to furnish the Company with copiestotal number of all Section 16(a) forms they file.
(2) Of these shares, as of December 31, 2016,2023, 712,026 shares remained available under the Company believes that all reports required by Section 16(a) of2019 Equity Incentive Plan, 5,624 shares remained available under the Exchange Act to be filed by our officers, directors2010 Non-Employee Directors’ Stock Award Plan and greater than 10% beneficial owners were complied with.35,946 shares remained available under the ESPP.
22
The following table shows certain information with respect to the compensation of all of our non-employee directors for the fiscal year ended December 31, 2016.2023.
| Name | Cash Compensation (1) | Option Awards ($) (2)(3)(4) | Total ($) | ||||||||||||||||||||
| Chad A. Johnson | $55,500 | $8,829 | $64,329 | ||||||||||||||||||||
| Thomas A. Raffin, M.D. | $78,000 | $8,829 | $86,829 | ||||||||||||||||||||
| An van Es-Johansson | $44,000 | $8,829 | $52,829 | ||||||||||||||||||||
| Lota S. Zoth | $60,500 | $8,829 | $69,329 | ||||||||||||||||||||
| Kevin Lalande | $45,500 | $8,829 | $54,329 | ||||||||||||||||||||
| Joseph S. McCracken | $47,500 | $8,829 | $56,329 | ||||||||||||||||||||
| (1) | Cash compensation is paid quarterly based on the annual amount of $40,000 for all non-employee directors with additional annual cash compensation of $22,000 for Lead Independent Director, $15,000, $12,000 and $8,000 for the Chairs of the Audit, Compensation and Nominating and Corporate Governance Committees, respectively; and $7,500, $5,500 and $4,000 for members of the Audit, Compensation and Nominating and Corporate Governance Committees, respectively. | |||||||||||||
| (2) | The assumptions we used in valuing options are described under the caption “Share-Based Compensation” in note 2 to our financial statements included in our Annual Report on Form 10-K filed on March 7, 2024. This column reflects the aggregate grant date fair value of options granted during the year indicated in accordance with FASB ASC Topic 718. | |||||||||||||
| (3) | The aggregate number of shares subject to stock option awards outstanding for each non-employee director as of December 31, 2023 are as follows: | |||||||||||||
| Option Awards | ||||||||||||||
| Chad A. Johnson | 22,374 | |||||||||||||
| Thomas A. Raffin, M.D. | 28,586 | |||||||||||||
| An van Es-Johansson | 15,415 | |||||||||||||
| Kevin Lalande | 15,370 | |||||||||||||
| Joseph S. McCracken | 15,370 | |||||||||||||
| Lota Zoth | 23,471 | |||||||||||||
| (4) | Reflects the grant date fair value of 3,345 options granted at an exercise price of $3.42, which was the per share closing price of our common stock on the NASDAQ Global Market on the date of grant. | |||||||||||||
| Name | Cash Compensation (1)(2) | Option Awards ($) (3)(4)(5) | Total ($) | |||||
| Paul R. Edick | $71,000 | $150,117 | $221,117 | |||||
| Paolo Pucci | $69,625 | $150,117 | $219,742 | |||||
| Thomas A. Raffin, M.D. | $99,167 | $150,117 | $249,284 | |||||
| Joseph Saluri | $69,000 | $150,117 | $219,117 | |||||
| Ernest J. Talarico, III | $75,000 | $150,117 | $225,117 | |||||
| Lota Zoth | $81,500 | $150,117 | $231,617 | |||||
| (1 | ) | Cash compensation paid quarterly based on the annual amount of $50,000 for all Non-Employee Directors with additional annual cash compensation of $30,000 for Lead Independent Director, $20,000, $17,500 and $10,000 for the Chairs of the Audit, Compensation and Nominating and Corporate Governance Committees, respectively; and $13,500, $11,500 and $7,500 for members of the Audit, Compensation and Nominating and Corporate Governance Committees, respectively. | ||
| (2 | ) | Effective following the Annual Meeting in May 2016 and based upon advice from Radford, the board increased the compensation paid for Lead Independent Director from $15,000 to $30,000 annually. This resulted in a proportional increase in the compensation for Dr. Raffin during the second quarter. | ||
| (3 | ) | The assumptions we used in valuing options are described under the caption “Share-Based Compensation” in note 2 to our financial statements included in our Annual Report on Form 10-K filed March 6, 2017. This column reflects compensation expense that would be recorded under FASB ASC topic 718 as stock-based compensation in our financial statements for the indicated year in connection with options we granted in the indicated year, disregarding the effects of any estimate of forfeitures related to service-based vesting. | ||
| (4 | ) | The number of stock awards and aggregate number of shares subject to stock option awards outstanding for each non-employee director as of December 31, 2016 are as follows: | ||
| Stock Awards | Option Awards | |||
| Paul R. Edick | — | 80,371 | ||
| Paolo Pucci | 3,337 | 41,378 | ||
| Thomas A. Raffin, M.D. | — | 169,656 | ||
| Joseph B. Saluri | — | 92,035 | ||
| Ernest J. Talarico, III | — | 144,174 | ||
| Lota Zoth | — | 79,181 | ||
| (5 | ) | Grant date fair value of 22,556 options granted in 2016 at an exercise price of $10.78, which was the per share closing price of our common stock on the NASDAQ Global Market on the date of grant. | ||
Non-Employee Director Compensation
The following compensation components are paid to our non-employee directors:
•Annual cash retainer fees;
•An equity grant upon initial election or appointment to our Board; and
•An annual equity grant.
Our non-employee director compensation program as in effect for the fiscal year ended December 31, 20162023 is as described below. For a description of our compensation program in effect for prior years, please refer to the proxy statement for our 20162022 Annual Meeting of stockholders. Under our program, each non-employee director was entitled to receive annual cash retainer fees in the amounts set forth below and were paid in cash quarterly on the first day of each quarter during their annual term commencing upon their election or re-election at each Annual Meeting of Stockholders. Such amounts were pro-rated for appointments made to our Board between our annual meetings.
23
| Annual retainer fee payable to all non-employee directors | $ | 50,000 | ||
| Additional annual retainer fee payable to the Lead Independent Director of our Board | $ | 30,000 | ||
| Additional annual retainer fee payable to our Audit Committee Chair | $ | 20,000 | ||
| Additional annual retainer fee payable to other Audit Committee members | $ | 13,500 | ||
| Additional annual retainer fee payable to our Compensation Committee Chair | $ | 17,500 | ||
| Additional annual retainer fee payable to other Compensation Committee members | $ | 11,500 | ||
| Additional annual retainer fee payable to our Nominating and Corporate Governance Committee Chair | $ | 10,000 | ||
| Additional annual retainer fee payable to other Nominating and Corporate Governance Committee members | $ | 7,500 | ||
| Director Compensation | 2023 | |||||||||||||
| Annual retainer fee payable to all non-employee directors | $ | 40,000 | ||||||||||||
| Additional annual retainer fee payable to the Lead Independent Director of our Board | $ | 22,000 | ||||||||||||
| Additional annual retainer fee payable to our Audit Committee Chair | $ | 15,000 | ||||||||||||
| Additional annual retainer fee payable to other Audit Committee members | $ | 7,500 | ||||||||||||
| Additional annual retainer fee payable to our Compensation Committee Chair | $ | 12,000 | ||||||||||||
| Additional annual retainer fee payable to other Compensation Committee members | $ | 5,500 | ||||||||||||
| Additional annual retainer fee payable to our Nominating and Corporate Governance Committee Chair | $ | 8,000 | ||||||||||||
| Additional annual retainer fee payable to other Nominating and Corporate Governance Committee members | $ | 4,000 | ||||||||||||
We also reimburse our directors, including our employee directors, for their reasonable expenses incurred in attending meetings of our Board and the committees of our Board. Other than reimbursement of any such reasonable expenses, our employee directors do not receive compensation for their service on our Board.
24
EXECUTIVE OFFICERS
Our executive officers are appointed by and serve at the direction of our BoardBoard. The following table lists the names and positions of Directors. There are no family relationships between our directors, director nominees, andcurrent executive officers. officers:
| Name | Age | Position(s) | |||||||||
| Richard J. Hawkins | 75 | Chief Executive Officer and Chair | |||||||||
| Lori D. Lawley | 40 | Chief Financial Officer | |||||||||
| John McKew, Ph.D. | 59 | President and Chief Scientific Officer | |||||||||
| Bradley J. Powers | 45 | General Counsel | |||||||||
Richard J. Link, Jr., M.D.Hawkins, , See Dr. Link’ssee Mr. Hawkin's biography in “Proposal"Proposal Number 1-Election1- Election of Directors.”"
John McKew, Ph.D., has served as our President since August 2021. Dr. McKew served as our Chief Scientific and Chief AdministrativeOperating Officer from March 2020 until August 2021 and served as the Chief Scientific Officer of Private Lumos from 2016 through the Merger in March 2020. From 2014 until 2016, Dr. McKew was V.P. of research for aTyr Pharma where he led research aimed at understanding and harnessing the therapeutic potential of tRNA synthetases. From 2010 until 2014, Dr. McKew worked for the NIH, during which time he served as a branch chief at the National Human Genome Research Institute Home (the “NHGRI”) from 2010 until 2013, and as the acting Scientific Director of the Division of Preclinical Innovation at the National Center for Advancing Translational Sciences (“NCATS”) from 2013 until 2014. His responsibilities included developing both the Therapeutics for Rare and Neglected Disease (“TRND”) and the Bridging Interventional Development Gaps (“BrIDGs”) programs. The department he led also included NCATS’s high throughput screening center and its Tox21 in vitro toxicology initiative. Before joining the NIH, Dr. McKew held a director level position at Wyeth Research in Cambridge, Massachusetts. Dr. McKew is also currently an Adjunct Associate Professor at the Boston University School of Medicine. Dr. McKew received a B.S. degree in chemistry and biochemistry from State University of New York at Stony Brook, a Ph.D. in organic chemistry from the University of California, Davis, and held post-doctoral research positions at the University of Geneva and Firmenich, SA.
Bradley J. Powers has served as our General Counsel since 2014.August 2015. Prior to joining the Company, Mr. HennemanPowers served for sixteen years at Integra Life Sciences, a publicly traded life sciences company. He joined Integra in 1998 as the General Counsel of Kinze Manufacturing, an agricultural equipment manufacturer in North America, since March 2013. Mr. Powers received a B.S. degree in biology and Chief Administrative Officer, was appointed Acting Chief Financial Officera M.S. degree in 2007bioinformatics and assumed that role permanently in 2008. In April 2014, he was named as Corporate Vice President and Chief Administrative Officer, concurrently with the appointment of a new Chief Financial Officer. During his 16 years at Integra, in addition to his responsibilities as Chief Financial Officer, he was responsible at various times for Integra's law department, corporate development, regulatory affairs, quality systems, clinical affairs, human resources, information systems and management of Integra’s surgical instruments business. Mr. Henneman currently serves on the Board of Directors of R1 RCM, Inc., a NASDAQ listed company that is a provider of revenue cycle management services to hospitals, SeaSpine Holdings Corporation, a NASDAQ listed company that is a spinal implant and orthobiologics company and Alafair Biosciences, a privately-held biomaterials company. Mr. Henneman received his A.B. in Politicscomputational biology from PrincetonIowa State University and a J.D. from theDrake University of Michigan Law School.
25
EXECUTIVE COMPENSATION
Summary Compensation Table
The following table sets forth information regarding compensation earned during the years ended December 31, 2023 and December 31, 2022, by the executive officers identified below, who are referred to in this proxy statement as our Chief Commercial Officer since 2015 and previously served as its Vice President of Business Development from 2013 to 2015. Mr. Wiley is responsible for the business development, commercialization and corporate communications strategies for all of our portfolio products. Prior to joining the Company, Mr. Wiley was the Principal and Founder of Boston BioConsulting, LLC, Senior Director of Oncology Marketing at Celgene Corporation & Gloucester Pharmaceuticals, and Associate Director of Oncology Marketing at Millennium Pharmaceuticals. Mr. Wiley also held multiple commercialization positions of increasing responsibility at Aventis/Sanofi Oncology from 1992-2004. Mr. Wiley received his B.A. from The Pennsylvania State University.“named executive officers.”
| Name and Principal Position | Year | Salary($) | Stock Awards ($)(1) | Option Awards ($)(2) | Non-Equity Incentive Plan Compensation ($)(3) | All Other Compensation ($)(4) | Total ($) | |||||||||||||||||||
| Richard J. Hawkins | 2023 | 582,624 | 15,840 | 82,677 | 224,311 | 47,417 | (5) | 952,869 | ||||||||||||||||||
| Chief Executive Officer | 2022 | 582,185 | 45,090 | 252,060 | 350,656 | 42,882 | (6) | 1,272,873 | ||||||||||||||||||
| John C. McKew | 2023 | 535,500 | 7,040 | 37,369 | 287,425 | 37,457 | (7) | 904,791 | ||||||||||||||||||
| President and Chief Scientific Officer | 2022 | 535,096 | 15,030 | 99,685 | 374,969 | 33,398 | (8) | 1,058,178 | ||||||||||||||||||
| Lori Lawley | 2023 | 391,000 | 3,520 | 28,026 | 109,480 | 37,749 | (9) | 569,775 | ||||||||||||||||||
| Chief Financial Officer | 2022 | 389,923 | 10,020 | 85,444 | 170,476 | 36,166 | (10) | 692,029 | ||||||||||||||||||
| (5) | Includes $917 in life insurance premiums. | |||||||
| (6) | Includes $632 in life insurance premiums. | |||||||
| (7) | Includes $2,748 in life insurance premiums. | |||||||
| (8) | Includes $2,196 in life insurance premiums. | |||||||
| (9) | Includes $1,749 in life insurance premiums. | |||||||
| (10) | Includes $1,416 in life insurance premiums. | |||||||
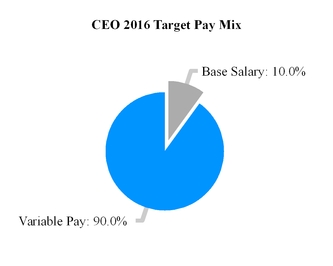
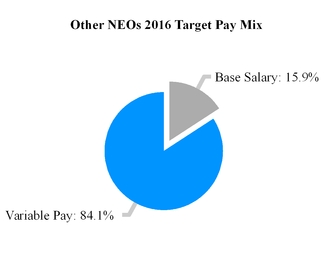
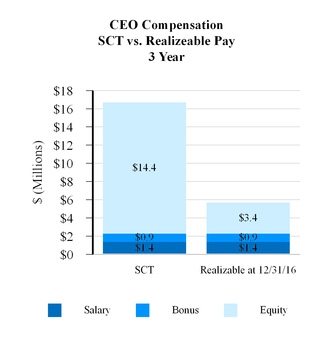
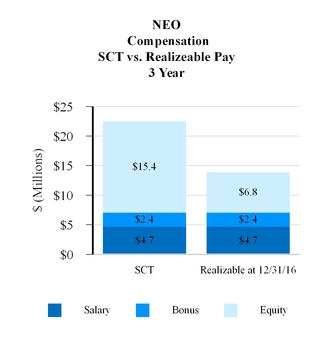
| Chief Executive Officer | 2016 SCT Compensation | Realizable Compensation at 12/31/16 | |
| Salary | $659,900 | $659,900 | |
| Bonus | $461,930 | # | $230,965 |
| Spot Bonus | $55,000 | $55,000 | |
| Restricted Stock | $1,619,772 | $479,449 | |
| Performance Based Restricted Stock Units | $974,281 | $72,096 | |
| Stock Options | $3,071,426 | $0 | |
| Total | $6,842,309 | $1,497,410 | |
Our executive officer compensation program is intended to achieve the following objectives:
| 12/31/15 Market Cap ($mm) | Number of Employees | ||||
| Peers | 75th percentile | 2,109.9 | 274 | ||
| Median | 1,182.3 | 194 | |||
| 25th percentile | 676.0 | 120 | |||
| NewLink Genetics Corporation | 1,047.5 | 130 | |||
| Rank | 37 | % | 34 | % | |
Base Salary
Base salary is the primary fixed element of our executive compensation program. We use base salary to compensate our executive officers for services rendered during the fiscal year, and to ensure that we remain competitive in attracting and retaining executive talent.
Upon joining us, each of our executive officers, with the exception of Dr. Link, received an offer letter that provided for an initial base salary. These initial base salaries are the product of negotiation with the executive, but we generally seek to establish salaries that we believe are commensurate with the salaries paid to industry peers with comparable qualifications, experience, responsibilities and performance at similar companies. Our Compensation Committee has also relied on its members’ collective experience in the marketplace for determining what they believe to be the market rate of salaries for executives of comparable companies.
Historically, we have not applied, nor do we intend to apply, specific formulas to determine base salary increases. Instead, we annually review corporate and individual performance. Our Compensation Committee examines numerous factors, including the executive’s expertise, seniority, position, functional role, level of responsibility and individual performance
26
during the previous year. Further, our Compensation Committee reviews peer and market data as provided by Radford.Setren & Associates.
Short-Term Cash Incentives
| NEO | 2015 Base Salary | 2016 Base Salary | % Change |
| Charles J. Link, Jr., M.D. | $640,700 | $659,900 | 3.0% |
| Nicholas N. Vahanian, M.D. | $531,800 | $574,300 | 8.0% |
| John B. Henneman, III | $365,200 | $423,600 | 16.0% |
| Brian Wiley | $319,600 | $370,700 | 16.0% |
| Carl Langren | $250,000 | $277,500 | 11.0% |
Our performance-based cash bonus program isincentives are designed to promote our interests and the interests of our stockholders by providingprovide executive officers with the opportunity to earn annual cash bonusesawards based upon the achievement of pre-specified corporate and individual performance objectives which align with and to assist us in attracting and retaining executive talent.support our business strategy.
Shortly before the end of each fiscal year, our Board determines the annual target bonus percentages for our executive officers for the upcoming fiscal year based on the recommendations of our Compensation Committee. Generally, each executive officer is eligible for a discretionary annual cash incentive payment up to a specified percentage of the executive officer’s salary. Our Board sets these annual target bonus percentages at levels that, upon achievement of the target percentage, are likely to result in cash bonus payments that our Board believes to be approximately the level paid to high-performing executives of comparable companies in the biopharmaceutical industry. The performance goals for each fiscal year are generally determined by the Board in the first quarter of such fiscal year, which may include corporate and/or individual performance objectives.
For 2016,2023, based upon recommendations of our Compensation Committee andwith the Radford Report,assistance of Setren & Associates, our Board establishedmaintained target bonus amounts for Dr. Link, Dr. Vahanian, Mr. Henneman,Hawkins, Mr. WileyMcKew and Mr. LangrenMs. Lawley equal to 70%55%, 50%, and 40%, 35% and 30% of their respective base salaries. Our Board reserved the ability to grant bonuses in excess of the executives’ target bonus percentages for extraordinary performance.
2023 Performance Objectives
The performance goals for each fiscal year are determined by the Board in the first quarter of each fiscal year, and typically include corporate and individual performance objectives which are tailored to each executive’s role and responsibilities.
For 2016, our NEOs2023, the Company had the following performancecorporate goals:
Clinical: •100% enrollment of randomized patients in OraGrowtH210 by end of Q2 and deliver top-line six month data by year end (15%) •OraGrowtH210 Trial six month AHV analysis from up to 24 subjects by year end (15%) •Bioavailability trial initiated by year end (10%) | |||||
CMC: •Drug products shipped and available at clinical site by Q4 2023 for relative bioavailability and other studies (10%) •Complete tech-transfer of drug substance manufacturing process and stability study initiation of 10 kg and 25 kg scale by state of Q4 2023 (5%) •Completion of analytical, quality and manufacturing process related factors for Phase 3 | 20% | ||||
Finance: •Secure financing through a combination of strategic transaction, regional license deal(s), financial sources and/or operational improvements to extend cash runway by an additional 6 to 12 months with Board approval | 30% | ||||
R&D and Business Development: •Successful negotiation of the license agreement and go/no-go decision by Board by the end of the year and generate scale up and | |||||
At the end of eachthe fiscal year, Dr. Link determines cash bonus recommendations for each of our executive officers, based on our corporate accomplishments and the individual’s performance and contributions to those accomplishments during the fiscal year. These recommendations are subjective determinations that may vary, from time to time, depending on our overall strategic objectives and the job responsibilities of each executive officer, but relate generally to factors such as development and progression of our existing product candidates, achievement of clinical and regulatory milestones, operational goals such as the expansion of
27
2023 Earned Bonuses
For 2023, the Compensation Committee continued to take a two-tiered approach at determining the amount of earned bonus for each executive based on the current company conditions as well as actual performance. First, the Compensation Committee looked at the performance of each executive against each of the stated performance goals. Secondly, in light of current events and biotech industry conditions as a whole, the Compensation Committee determined it to be in the best interests of the company and its stockholders to use the bonus earning as a retention device in efforts to retain key personnel. Additionally, the Compensation Committee, at its discretion, determined that Dr. McKew should receive a supplemental bonus because he provided contributions to the Company that are expected to increase stockholder value. Dr. McKew's contributions include extensive leadership during the analysis and reporting of our Compensation Committee,top line data analysis for our OraGrowtH210 and OraGrowtH212 Trials, as well as considerable contributions and guidance throughout the non-employee directors on our Board then makeprocess of acquiring patent protection for a final decision regarding cash bonus payments, if any, for the year. Whether or not a bonus is paid for any year is solely within the discretionnovel formulation of our Board.LUM-201 to extend exclusivity to 2042.
For 2023, the Compensation Committee determined that executives had achieved 50% of their bonus objectives,performance with respect to the stated goals would result in a 70% payout, and bonuses were paid accordingly. Separately, in recognition of the NEOs efforts in the successful completion of the restructuring following the failure of the Phase 3 algenpantucel-L trials, our executives received a spot bonus.
Final bonus payouts for 20162023 performance were as follows:
| Executive | 2023 Target Bonus | 2023 Earned Bonus | Supplemental Bonus(4) | Total Bonus Payout | ||||||||||||||||||||||
%(1) | $ | %(2) | %(3) | $ | ||||||||||||||||||||||
| Richard J. Hawkins | 55 | $320,443 | 70 | 38.5 | $224,311 | $0 | $224,311 | |||||||||||||||||||
| John C. McKew | 50 | $267,750 | 70 | 35.0 | $187,425 | $100,000 | $287,425 | |||||||||||||||||||
| Lori Lawley | 40 | $156,400 | 70 | 28.0 | $109,480 | $0 | $109,480 | |||||||||||||||||||
(1) Target bonus percentage represents a percentage of base salary.
| NEO | Target Bonus | Bonus Awarded | Bonus Amount | Spot Bonus | Total 2016 Bonus |
| Charles J. Link, Jr., M.D. | $461,930 | 50% | $230,965 | $55,000 | $285,965 |
| Nicholas N. Vahanian, M.D. | $287,150 | 50% | $143,575 | $43,000 | $186,575 |
| John B. Henneman, III | $169,440 | 50% | $84,720 | $26,250 | $110,970 |
| Brian Wiley | $129,745 | 50% | $64,873 | $19,525 | $84,398 |
| Carl Langren | $83,250 | 50% | $41,625 | $12,625 | $54,250 |
(3) Represents bonus percentage actually awarded.
Long-Term Equity Compensation
Equity incentives represent the largest at-risk element of our executive compensation program. Our equity incentives are designed to align the interests of our executive officers with those of our stockholders by creating an incentive for our executive officers to maximize stockholder value and to remain employed with us despite a competitive labor market.
In 2023, target long-term incentive awards were delivered in the form of time-based stock awards grants are made to our existing executive officers during the first quarter of each fiscal year, and these annualoptions, performance-based stock awards consist of restricted stock units, or RSUs, and stock options. The number of stock awards is based on a targeted percentage of our outstanding shares based on a particular percentage of our peer group and are granted in relative proportion of 35% and 65%, calculated based on the fair value of the awards on the date of grant. Stock options and RSUs typically vest monthly over 48 months. Beginning in 2016, in an effortRSUs. The Compensation Committee looked to more closely align compensation tothe successful nature of meeting company performance, the Company issued performance restricted stock units, or pRSUs, that vest only under previously determined specific conditions. The pRSUs granted in 2016 shall vest on the following schedule:
The awards had the following vesting and performance criteria:
Time-vested Options - These options vest and become exercisable in a series of 48 successive, substantially equal monthly installments.
Performance-based Options - These options vest and become exercisable in three substantially equal installments on the first of the month following an increase of closing stock unitsprice, as follows:defined as the closing stock price on Nasdaq Stock Market by at least 50%, 100% and 150%, respectively, above the closing stock price on December 31, 2022, when measured over 30 consecutive calendar days, provided such increase occurs within two years of the date of grant, otherwise such options shall be cancelled.
| NEO | Options (#) | RSUs (#) | pRSUs (#)(1) |
| Charles J. Link, Jr., M.D. | 133,435 | 46,639 | 28,053 |
| Nicholas N. Vahanian, M.D. | 84,275 | 29,456 | 19,637 |
| John B. Henneman, III | 196,351 | 16,201 | 9,819 |
| Brian Wiley | 48,259 | 6,382 | 7,013 |
| Carl Langren | 28,989 | 3,142 | 1,122 |
| (1) | As a result of the performance conditions described in detail above, in connection with the negative result from our IMPRESS trial, 75% of each of these pRSUs were forfeited in 2016. | ||
On January 3, 2017,February 1, 2023, our Compensation Committee granted executives stock options, but did not awardthe following equity awards to our executives any RSUs or pRSUs. For Dr. Link, Dr. Vahanian, and Mr. Henneman, 50% of option grants will vest monthly over 48 monthly installments, as in past years. The other 50% of options grants will vest contingent on the achievement of performance conditions as follows:
28
| Executive | Time-vesting Stock Options (#) | Performance-based Stock Options (#) | RSUs (#) | ||||||||
| Richard J. Hawkins | 17,700 | 17,700 | 4,500 | ||||||||
| John C. McKew | 8,000 | 8,000 | 2,000 | ||||||||
| Lori Lawley | 6,000 | 6,000 | 1,000 | ||||||||
Anti-Hedging and Anti-Pledging Policies
We have adopted a Policy on Stock Trading by Employees, Officers and Directors. That policy expressly prohibitprohibits our employees and directors from: (i) engaging in hedging transactions or (ii) pledging our stock as collateral.
Federal Tax Considerations Under Sections 162(m) and 409A
Section 162(m) limits ourof the Internal Revenue Code generally disallows public companies a tax deduction for federal income tax purposes to not more thanof remuneration in excess of $1 million of compensation paid to specifiedthe chief executive officer and certain other highly compensated executive officers in a calendarany taxable year. While our Compensation above $1 million may be deducted ifCommittee considers tax deductibility as one of the factors in determining executive compensation, the Compensation Committee believes that we should retain the flexibility to provide compensation to our named executive officers that is not fully tax deductible when it is performance-based compensation within the meaning of Section 162(m). With the exception of fiscal year 2014, we have had a history of operating lossesbelieves that such payments are appropriate to attract and expectretain executive talent or meet other business objectives. Our Compensation Committee intends to continue to incur operating losses for the foreseeable future. These net operating loss carryforwards would have the effect of offsetting certain future taxable gains, and as such, we generally do not consider the tax implications ofcompensate our executive compensation programs to be meaningful to our operating or financial results. To maintain flexibility in compensating ournamed executive officers in a manner designedconsistent with the best interests of our Company and our stockholders even if any portion of such compensation is non-deductible.
In addition to promote our compensation objectives, ourconsidering the tax consequences, the Compensation Committee has not yet established a policy for determining which forms of incentive compensation awarded to our executive officers will be designed to qualify as performance-based compensation.
Accounting Considerations
We account for equity compensation paid to our employees in accordance with Accounting Standards Codification, or ASC, topic 718, which requires us to measure and recognize compensation expense in our financial statements for all share-based payments based upon an estimate of their fair value over the service period of the award. We record cash compensation as an expense at the time the obligation is incurred.
401(k) Plan
Our employees, including our executive officers, are eligible to participate in our 401(k) plan. Our 401(k) plan is intended to qualify as a tax qualified plan under Section 401 of the Code. Pursuant to the terms of our 401(k) plan, we provide a non-elective employer contribution of up to 3%5% of each participant’s eligible compensation, or the Safe Harbor Contribution, with a possibility of additional discretionary contributions. In January 2016, our Board approved discretionary employer contributions in amounts that, when added to the Safe Harbor Contributions, amounted to 4.5% of total potential 2016 cash compensation for each member of the senior management team and 5% of total 2016 cash compensation for all other eligible employees.
Other Benefits and Perquisites
We pay at least a portion of the premiums for medical insurance, dental insurance, life insurance and accidental death and dismemberment insurance benefits to all full-time employees, including our executive officers. These benefits are available to all employees, subject to applicable laws.
| Name and Principal Position | Year | Salary ($) | Bonus (1) | Stock Awards (2)(3)($) | Option Awards(4)($) | Non-Equity Incentive Plan Compensation ($)(5) | All Other Compensation ($)(6) | Total ($) | ||||||||
| Charles J. Link, Jr., M.D. | 2016 | 659,900 | 55,000 | 2,594,053 | 3,071,426 | 230,965 | 93,500 | (7) | 6,704,844 | |||||||
| Chief Executive & Scientific Officer | 2015 | 640,700 | — | 1,964,250 | 3,825,722 | 422,862 | 102,960 | (8) | 6,956,494 | |||||||
| 2014 | 573,200 | — | 758,990 | 1,354,861 | 515,880 | 88,277 | (9) | 3,291,208 | ||||||||
| Nicholas N. Vahanian, M.D. | 2016 | 574,300 | 43,000 | 1,705,000 | 1,939,848 | 143,575 | 44,200 | (10) | 4,449,923 | |||||||
| President & Chief Medical Officer | 2015 | 531,800 | — | 1,091,250 | 2,167,018 | 305,785 | 61,706 | (11) | 4,157,559 | |||||||
| 2014 | 453,600 | — | 523,810 | 887,667 | 340,200 | 47,530 | (12) | 2,252,807 | ||||||||
| John B. Henneman, III | 2016 | 423,600 | 26,250 | 903,675 | 2,153,813 | 84,720 | 26,729 | 3,618,787 | ||||||||
| Chief Financial Officer & Secretary | 2015 | 365,200 | — | 87,300 | 173,896 | 167,992 | 23,994 | 818,382 | ||||||||
| 2014 | 90,000 | — | 936,108 | 2,990,967 | — | — | 4,017,075 | |||||||||
| Brian Wiley | 2016 | 370,700 | 19,525 | 465,208 | 637,668 | 64,873 | 22,755 | 1,580,729 | ||||||||
| Chief Commercial Officer | 2015 | 319,600 | — | 323,010 | 626,027 | 95,880 | 18,697 | 1,383,214 | ||||||||
| 2014 | 262,500 | — | 121,866 | 338,917 | 118,125 | 15,356 | 856,764 | |||||||||
| Carl Langren | 2016 | 277,500 | 12,625 | 148,089 | 346,385 | 41,625 | 16,588 | 842,812 | ||||||||
| Vice President of Finance | 2015 | 250,000 | — | 222,615 | 441,290 | 75,000 | 14,625 | 1,003,530 | ||||||||
Estimated Future Payouts under Non-Equity Incentive Plan Awards(1) | All Other Stock Awards: Number of Units (#) (2) | All other Option Awards: Number of Securities Underlying Options (#)(3) | Exercise or Base Price of Option Awards ($/Sh)(4) | Grant Date Fair Value of Option and Stock Awards(5) | |||||||||
| Name | Grant Date | Threshold ($) | Target ($) | Maximum ($) | |||||||||
| Charles J. Link, Jr., M.D. | 1/4/2016 | — | 461,930 | 461,930 | |||||||||
| 1/4/2016 | 46,639 | $1,619,772 | |||||||||||
| 1/4/2016 | 28,053 | (6) | $974,281 | ||||||||||
| 1/4/2016 | 133,435 | 34.73 | $3,071,426 | ||||||||||
| Nicholas N. Vahanian, M.D. | 1/4/2016 | — | 287,150 | 287,150 | |||||||||
| 1/4/2016 | 29,456 | $1,023,007 | |||||||||||
| 1/4/2016 | 19,637 | (6) | $681,993 | ||||||||||
| 1/4/2016 | 84,275 | 34.73 | $1,939,848 | ||||||||||
| John B. Henneman, III | 1/4/2016 | — | 169,440 | 169,440 | |||||||||
| 1/4/2016 | 16,201 | $562,661 | |||||||||||
| 1/4/2016 | 9,819 | (6) | $341,014 | ||||||||||
| 1/4/2016 | 46,351 | 34.73 | $1,066,910 | ||||||||||
| 8/9/2016 | 150,000 | 10.78 | $1,086,903 | ||||||||||
| Brian Wiley | 1/4/2016 | — | 129,745 | 129,745 | |||||||||
| 1/4/2016 | 6,382 | $221,647 | |||||||||||
| 1/4/2016 | 7,013 | (6) | $243,561 | ||||||||||
| 1/4/2016 | 18,259 | 34.73 | $420,287 | ||||||||||
| 8/9/2016 | 30,000 | 10.73 | $217,381 | ||||||||||
| Carl Langren | 1/4/2016 | — | 83,250 | 83,250 | |||||||||
| 1/4/2016 | 3,142 | $109,122 | |||||||||||
| 1/4/2016 | 1,122 | (6) | $38,967 | ||||||||||
| 1/4/2016 | 8,989 | 34.73 | $203,677 | ||||||||||
| 8/9/2016 | 20,000 | 10.78 | $142,708 | ||||||||||
Employment Agreements
We have entered into employment agreements with each of the NEOs.named executive officers. The material terms of the agreements that were in effect during fiscal 20162023 for the NEOsnamed executive officers are summarized below. Each of these agreements also contains severance and change of control provisions discussed under the heading "Potential“Potential Payments Upon Termination or Change in Control"Control” beginning on page 4532 of this proxy statement.
29
Employment Agreement with Dr. CharlesRichard J. Link, Jr.Hawkins
Pursuant to the employment agreement between us and Dr. Link that was amended and restated on January 4, 2016, Dr. LinkMr. Hawkins dated March 27, 2020, Mr. Hawkins earns an annual base salary, which is subject to annual review and adjustment by our Board. For 2016, Dr. Link2023, Mr. Hawkins earned an annual base salary of $659,900. Dr. Link$582,624. Mr. Hawkins is also eligible to receive an annual performance bonus based on his achievement of certain milestones and performance objectives. For 2016, Dr. Link’sIn 2023, Mr. Hawkins’ target bonus was set at 70%55% of his annual base salary.salary, which was the same level as 2022.
The employment agreement with Dr. LinkMr. Hawkins also provides that his employment with us is at-will and may be altered or terminated by either Dr. LinkMr. Hawkins or us at any time. However, if we terminate Dr. Link’sMr. Hawkins’ employment without just cause or if he resigns for good reason (other than in connection with a change in control of us), as long as Dr. LinkMr. Hawkins executes a general release in favor of us, he will be entitled to receive certain payments and other benefits, which are described in more detail under the heading “Potential Payments Upon Termination or Change in Control” beginning on page 45 of this proxy statement.Control.”
The employment agreement with Dr. LinkMr. Hawkins further provides that if we (or any surviving or acquiring corporation) terminate Dr. Link’sMr. Hawkins’ employment without just cause or if he resigns for good reason within one month prior to or 13 months following the effective date of a change in control, as long as Dr. LinkMr. Hawkins executes a general release in favor of us (or any surviving or acquiring corporation), he will be entitled to receive certain payments and other benefits, which are described in more detail under the heading “Potential Payments Upon Termination or Change in Control” beginning on page 45 of this proxy statement.Control.”
Employment Agreement with Dr. Nicholas N. VahanianJohn C. McKew
Pursuant to the employment agreement between us and Dr. Vahanian that wasMcKew dated March 27, 2020, as amended and restated on January 4, 2016,August 1, 2021, Dr. VahanianMcKew earns an annual base salary, which is subject to annual review and adjustment by our Board. For 2016,2023, Dr. VahanianMcKew earned an annual base salary of $574,300.$535,500. Dr. VahanianMcKew is also eligible to receive an annual performance bonus based on his achievement of certain milestones and performance objectives. For 2016,In 2023, Dr. Vahanian’sMcKew’s target bonus was set at 50% of his annual base salary.salary, which was the same level as 2022.
The employment agreement with Dr. VahanianMcKew also provides that his employment with us is at-will and may be altered or terminated by either Dr. VahanianMcKew or us at any time. However, if we terminate Dr. Vahanian’sMcKew’s employment without just cause or if he resigns for good reason (other than in connection with a change in control of us), as long as Dr. VahanianMcKew executes a general release in favor of us, he will be entitled to receive certain payments and other benefits, which are described in more detail under the heading “Potential Payments Upon Termination or Change in Control” beginning on page 45 of this proxy statement.Control.”
The employment agreement with Dr. VahanianMcKew further provides that if we (or any surviving or acquiring corporation) terminate Dr. Vahanian’sMcKew’s employment without just cause or if he resigns for good reason within one month prior to or 13 months following the effective date of a change in control, as long as Dr. VahanianMcKew executes a general release in favor of us (or any surviving or acquiring corporation), he will be entitled to receive certain payments and other benefits, which are described in more detail under the heading “Potential Payments Upon Termination or Change in Control” beginning on page 45 of this proxy statement.Control.”
Employment Agreement with John B. Henneman, IIILori Lawley
Pursuant to the employment agreement Mr. Hennemanbetween us and Ms. Lawley dated September 30, 2019, as amended June 30, 2021, Ms. Lawley earns an annual base salary, which is subject to annual review and adjustment by our Board. In 2016, Mr. HennemanFor 2023, Ms. Lawley earned an annual base salary of $423,600. Mr. Henneman$391,000. Ms. Lawley is also eligible to receive an annual performance bonus based on hisher achievement of certain milestones and performance objectives. In 2016, Mr. Henneman’sFor 2023, Ms. Lawley’ target bonus was set at 40% of hisher annual base salary.salary, which was the same level as 2022.
The employment agreement with Mr. HennemanMs. Lawley also provides that hisher employment with us is at-will and may be altered or terminated by either Mr. HennemanMs. Lawley or us at any time. However, if we terminate Mr. Henneman’sMs. Lawley’ employment without just cause or if heshe resigns for good reason (other than in connection with a change in control of us), as long as Mr. HennemanMs. Lawley executes a general release in favor of us, heshe will be entitled to receive certain payments and other benefits, which are described in more detail under the heading “Potential Payments Upon Termination or Change in Control” beginning on page 45 of this proxy statement.Control.”
The employment agreement with Mr. HennemanMs. Lawley further provides that if we (or any surviving or acquiring corporation) terminate Mr. Henneman’sMs. Lawley’ employment without just cause or if heshe resigns for good reason within one month prior to or 13 months following the effective date of a change in control, as long as Mr. HennemanMs. Lawley executes a general release in favor of us (or any surviving or acquiring corporation), he will be entitled to receive certain payments and other benefits, which are described in more detail under the heading “Potential Payments Upon Termination or Change in Control” beginning on page 45 of this proxy statement.Control.”
30
Confidential Information and Inventions Agreement
Each of our named executive officers has entered into a form agreement with respect to confidential information and assignment of inventions. Among other things, this agreement obligates each named executive officer to refrain from disclosing any of our confidential information received during the course of employment and, with some exceptions, to assign to us any inventions conceived or developed during the course of employment.
| Name | Number of Shares Acquired on Exercise of Stock Options(#) | Number of Shares Acquired on RSU Vest(#) | Value Realized on Exercise or Vest ($) | |||
| Charles J. Link, Jr. M.D. | — | — | ||||
| Chief Executive & Scientific Officer | 17,000 | $618,630 | ||||
| Nicholas N. Vahanian, M.D. | — | — | ||||
| President & Chief Medical Officer | 10,000 | $363,900 | ||||
| John B. Henneman, III | — | — | ||||
| Chief Financial Officer & Secretary | 10,697 | $171,354 | ||||
| Brian Wiley | — | — | ||||
| Chief Commercial Officer | 3,275 | $119,177 | ||||
| Carl Langren | — | — | ||||
| Vice President of Finance | 2,700 | $98,253 | ||||
Outstanding Equity Awards at December 31, 20162023
The following table provides information about outstanding stock options and restricted stock unitsRSUs held by each of our named executive officers at December 31, 2016.2023. All of these options or restricted stock unitsRSUs were granted under our 2000 Equity Incentive Plan or our 2009 Equity Incentive2019 Plan.
| Option Awards | Stock Awards | |||||||||||||||||||||||||||||||
| Number of Shares Underlying Unexercised Options(1) | ||||||||||||||||||||||||||||||||
| Name | (#) Exercisable | (#) Unexercisable (2) | Grant Date | Option Exercise Price | Option Expiration Date | Number of Shares or Units of Stock That Have Not Vested (#)(3) | Market Value of Shares or Units of Stock That Have Not Vested ($)(4) | |||||||||||||||||||||||||
| Richard J. Hawkins | 19,624 | — | 6/27/2019 | $ | 1.84 | 6/26/2029 | — | — | ||||||||||||||||||||||||
| 50,824 | 11,250 | 4/1/2020 | $ | 7.87 | 3/31/2030 | — | — | |||||||||||||||||||||||||
| 72,926 | — | 4/1/2020 | $ | 7.87 | 3/31/2030 | — | — | |||||||||||||||||||||||||
| 4/1/2020 | 6,250 | 19,875 | ||||||||||||||||||||||||||||||
| 20,169 | 8,306 | 2/1/2021 | $ | 17.35 | 1/31/2031 | — | — | |||||||||||||||||||||||||
| 2/1/2021 | 1,165 | 3,705 | ||||||||||||||||||||||||||||||
| 16,225 | 19,175 | 2/1/2022 | $ | 10.02 | 1/31/2032 | — | — | |||||||||||||||||||||||||
| 2/1/2022 | 3,375 | 10,733 | ||||||||||||||||||||||||||||||
| 3,687 | 14,013 | 2/1/2023 | $ | 3.52 | 1/31/2033 | |||||||||||||||||||||||||||
| 5,900 | (5) | 2/1/2023 | $ | 3.52 | 1/31/2025 | |||||||||||||||||||||||||||
| 5,900 | (6) | 2/1/2023 | $ | 3.52 | 1/31/2025 | |||||||||||||||||||||||||||
| 5,900 | (7) | 2/1/2023 | $ | 3.52 | 1/31/2025 | |||||||||||||||||||||||||||
| 2/1/2023 | 4,500 | 14,310 | ||||||||||||||||||||||||||||||
| John C. McKew | 66,697 | — | 7/11/2016 | $ | 4.82 | 7/10/2026 | — | — | ||||||||||||||||||||||||
| 14,993 | — | 7/11/2016 | $ | 4.82 | 7/10/2026 | — | — | |||||||||||||||||||||||||
| 9,583 | — | 1/18/2018 | $ | 2.45 | 1/17/2028 | — | — | |||||||||||||||||||||||||
| 56 | — | 8/29/2018 | $ | 2.45 | 8/28/2028 | — | — | |||||||||||||||||||||||||
| 6,485 | — | 8/29/2018 | $ | 2.45 | 8/28/2028 | — | — | |||||||||||||||||||||||||
| 58,329 | 5,417 | 4/1/2020 | $ | 7.87 | 3/31/2030 | — | — | |||||||||||||||||||||||||
| 1,254 | — | 4/1/2020 | $ | 7.87 | 3/31/2030 | — | — | |||||||||||||||||||||||||
| 4/1/2020 | 3,250 | 10,335 | ||||||||||||||||||||||||||||||
| 7,083 | 2,917 | 2/1/2021 | $ | 17.35 | 1/31/2031 | — | — | |||||||||||||||||||||||||
| 9,332 | 3,843 | 2/1/2021 | $ | 17.35 | 1/31/2031 | — | — | |||||||||||||||||||||||||
| 5,000 | — | (8) | 2/1/2021 | $ | 17.35 | 1/31/2031 | — | — | ||||||||||||||||||||||||
| 5,000 | — | (9) | 2/1/2021 | $ | 17.35 | 1/31/2031 | — | — | ||||||||||||||||||||||||
| 2/1/2021 | 539 | 1,714 | ||||||||||||||||||||||||||||||
| 8/1/2021 | 10,000 | 31,800 | ||||||||||||||||||||||||||||||
| 6,416 | 7,584 | 2/1/2022 | $ | 10.02 | 1/31/2032 | — | — | |||||||||||||||||||||||||
| 2/1/2022 | 1,125 | 3,578 | ||||||||||||||||||||||||||||||
| 1,666 | 6,334 | 2/1/2023 | $ | 3.52 | 1/31/2033 | |||||||||||||||||||||||||||
| 2,667 | (5) | 2/1/2023 | $ | 3.52 | 1/31/2025 | |||||||||||||||||||||||||||
| 2,667 | (6) | 2/1/2023 | $ | 3.52 | 1/31/2025 | |||||||||||||||||||||||||||
| 2,666 | (7) | 2/1/2023 | $ | 3.52 | 1/31/2025 | |||||||||||||||||||||||||||
| 2/1/2023 | 2,000 | 6,360 | ||||||||||||||||||||||||||||||
31
| Number of Shares Underlying Unexercised Options(1) | Number of Shares Underlying Unvested Restricted Stock Units (2) | Option Grant Date | Option Exercise Price | Option Expiration Date | ||||||||
| (#) Exercisable | (#) Unexercisable (3) | |||||||||||
| Charles J. Link, Jr., M.D. | 2,564 | (4)(8) | — | 6/1/2007 | $0.80 | 1/21/2019 | ||||||
| 264,474 | (4)(6) | — | 6/1/2007 | $4.20 | 5/13/2019 | |||||||
| 468,037 | (4)(6) | — | 12/4/2009 | $2.10 | 5/13/2019 | |||||||
| 428,571 | — | 1/1/2009 | $2.97 | 12/4/2019 | ||||||||
| 50,792 | — | 4/14/2011 | $7.00 | 4/13/2021 | ||||||||
| 44,446 | — | 4/14/2011 | $7.00 | 4/13/2021 | ||||||||
| 9,385 | — | 1/19/2012 | $6.87 | 1/18/2022 | ||||||||
| 130,615 | — | 1/19/2012 | $6.87 | 1/18/2022 | ||||||||
| 6,782 | 3,230 | 1/14/2013 | $11.79 | 1/13/2023 | ||||||||
| 144,988 | — | 1/14/2013 | $11.79 | 1/13/2023 | ||||||||
| — | 5,162 | 1/2/2014 | $21.38 | 1/1/2024 | ||||||||
| 79,296 | (5) | 24,292 | 1/2/2014 | $21.38 | 1/1/2024 | |||||||
| 11,500 | 1/2/2014 | $21.38 | ||||||||||
| — | (5) | 3,471 | 1/2/2015 | $43.65 | 1/1/2025 | |||||||
| 68,520 | (5) | 71,009 | 1/2/2015 | $43.65 | 1/1/2025 | |||||||
| 33,750 | 1/2/2015 | $43.65 | ||||||||||
| — | (5) | 2,780 | 1/4/2016 | $34.73 | 1/3/2026 | |||||||
| 30,578 | (5) | 100,077 | 1/4/2016 | $34.73 | 1/3/2026 | |||||||
| 46,639 | 1/4/2016 | $34.73 | ||||||||||
| 7,013 | (9) | 1/4/2016 | $34.73 | |||||||||
| Nicholas N. Vahanian, M.D. | 145,451 | (4)(7) | — | 6/1/2007 | $2.10 | 5/12/2019 | ||||||
| 59,444 | — | 6/1/2007 | $2.10 | 5/12/2019 | ||||||||
| 45,160 | — | 12/4/2009 | $2.97 | 12/3/2019 | ||||||||
| 335,792 | — | 12/4/2009 | $2.97 | 12/3/2019 | ||||||||
| 190,476 | — | 3/3/2010 | $3.07 | 3/2/2020 | ||||||||
| 14,286 | — | 4/14/2011 | $7.00 | 4/13/2021 | ||||||||
| 28,571 | — | 4/14/2011 | $7.00 | 4/13/2021 | ||||||||
| 16,159 | — | 1/19/2012 | $6.87 | 1/18/2022 | ||||||||
| 63,841 | — | 1/19/2012 | $6.87 | 1/18/2022 | ||||||||
| 7,510 | 1,875 | 1/14/2013 | $11.79 | 1/13/2023 | ||||||||
| 80,615 | — | 1/14/2013 | $11.79 | 1/13/2023 | ||||||||
| — | (5) | 5,128 | 1/2/2014 | $21.38 | 1/1/2024 | |||||||
| 51,953 | (5) | 14,169 | 1/2/2014 | $21.38 | 1/1/2024 | |||||||
| 7,500 | 1/2/2014 | $21.38 | ||||||||||
| — | (5) | 3,251 | 1/2/2015 | $43.65 | 1/1/2025 | |||||||
| 38,812 | (5) | 38,937 | 1/2/2015 | $43.65 | 1/1/2025 | |||||||
| 18,750 | 1/2/2015 | $43.65 | ||||||||||
| — | (5) | 2,513 | 1/4/2016 | $34.73 | 1/3/2026 | |||||||
| 19,313 | (5) | 62,449 | 1/4/2016 | $34.73 | 1/3/2026 | |||||||
| 29,456 | 1/4/2016 | $34.73 | ||||||||||
| 4,909 | (9) | 1/4/2016 | $34.73 | |||||||||
| John B. Henneman, III | 8,714 | 8,714 | 10/1/2014 | $22.95 | 9/30/2024 | |||||||
| 104,629 | 87,193 | 10/1/2014 | $22.95 | 9/30/2024 | ||||||||
| 20,395 | 10/1/2014 | $22.95 | ||||||||||
| — | (5) | 136 | 1/2/2015 | $43.65 | 1/1/2025 | |||||||
| 3,114 | (5) | 3,250 | 1/2/2015 | $43.65 | 1/1/2025 | |||||||
| 1,500 | 1/2/2015 | $43.65 | ||||||||||
| — | (5) | 3,674 | 1/4/2016 | $34.73 | 1/3/2026 | |||||||
| 10,622 | (5) | 32,055 | 1/4/2016 | $34.73 | 1/3/2026 | |||||||
| 16,666 | (5) | 133,334 | 8/9/2016 | $10.78 | 8/8/2026 | |||||||
| 16,201 | 1/4/2016 | $34.73 | ||||||||||
| 2,455 | (9) | 1/4/2016 | $34.73 | |||||||||
| Brian Wiley | 8,481 | 4,250 | 1/14/2013 | $11.79 | 1/13/2023 | |||||||
| 86,269 | — | 1/14/2013 | $11.79 | 1/13/2023 | ||||||||
| — | (5) | 2,896 | 1/2/2014 | $21.38 | 1/1/2024 | |||||||
| 19,687 | (5) | 4,417 | 1/2/2014 | $21.38 | 1/1/2024 | |||||||
| 2,850 | 1/2/2014 | $21.38 | ||||||||||
| — | (5) | 2,503 | 1/2/2015 | $43.65 | 1/1/2025 | |||||||
| 11,212 | (5) | 9,685 | 1/2/2015 | $43.65 | 1/1/2025 | |||||||
| 5,550 | 1/2/2015 | $43.65 | ||||||||||
| — | (5) | 2,647 | 1/4/2016 | $34.73 | 1/3/2026 | |||||||
| 4,184 | (5) | 11,428 | 1/4/2016 | $34.73 | 1/3/2026 | |||||||
| 3,333 | (5) | 26,667 | 8/9/2016 | $10.78 | 8/8/2026 | |||||||
| 6,382 | 1/4/2016 | $34.73 | ||||||||||
| 1,753 | (9) | 1/4/2016 | $34.73 | |||||||||
| Carl Langren | 9,523 | — | 12/14/2007 | $2.10 | 12/13/2017 | |||||||
| 2,380 | — | 3/3/2010 | $3.07 | 3/2/2020 | ||||||||
| 2,564 | (8) | — | 1/8/2011 | $0.80 | 1/20/2019 | |||||||
| 16,666 | — | 4/14/2011 | $7.00 | 4/13/2021 | ||||||||
| 18,076 | — | 10/19/2011 | $7.00 | 10/18/2021 | ||||||||
| 5,733 | — | 10/19/2011 | $7.00 | 10/18/2021 | ||||||||
| 10,944 | — | 1/19/2012 | $6.87 | 1/18/2022 | ||||||||
| 4,056 | — | 1/19/2012 | $6.87 | 1/18/2022 | ||||||||
| 10,198 | 417 | 1/14/2013 | $11.79 | 1/13/2023 | ||||||||
| 9,385 | — | 1/14/2013 | $11.79 | 1/13/2023 | ||||||||
| 1,916 | (5) | 5,010 | 1/2/2014 | $21.38 | 1/1/2024 | |||||||
| 17,771 | (5) | 2,303 | 1/2/2014 | $21.38 | 1/1/2024 | |||||||
| 2,850 | 1/2/2014 | $21.38 | ||||||||||
| — | (5) | 2,355 | 1/2/2015 | $43.65 | 1/1/2025 | |||||||
| 7,810 | (5) | 6,135 | 1/2/2015 | $43.65 | 1/1/2025 | |||||||
| 3,825 | 1/2/2015 | $43.65 | ||||||||||
| — | (5) | 2,435 | 1/4/2016 | $34.73 | 1/3/2026 | |||||||
| 2,059 | (5) | 4,495 | 1/4/2016 | $34.73 | 1/3/2026 | |||||||
| — | (5) | 660 | 8/9/2016 | $10.78 | 8/8/2026 | |||||||
| 2,222 | (5) | 17,188 | 8/9/2016 | $10.78 | 8/8/2026 | |||||||
| 3,142 | 1/4/2016 | $34.73 | ||||||||||
| 280 | (9) | 1/4/2016 | $34.73 | |||||||||
| Lori Lawley | ||||||||||||||||||||||||||||||||
| 2,776 | — | 8/1/2018 | $ | 28.53 | 7/31/2028 | — | — | |||||||||||||||||||||||||
| 2,257 | — | 3/1/2019 | $ | 16.20 | 2/28/2029 | — | — | |||||||||||||||||||||||||
| 520 | — | 3/1/2019 | $ | 16.20 | 2/28/2029 | — | — | |||||||||||||||||||||||||
| 463 | — | 3/1/2019 | $ | 16.20 | 2/28/2029 | — | — | |||||||||||||||||||||||||
| 694 | — | 3/1/2019 | $ | 16.20 | 2/28/2029 | — | — | |||||||||||||||||||||||||
| 1,282 | — | (10) | 7/31/2019 | $ | 15.93 | 7/30/2026 | — | — | ||||||||||||||||||||||||
| 22,916 | 2,084 | 4/1/2020 | $ | 7.87 | 3/31/2030 | — | — | |||||||||||||||||||||||||
| 4/1/2020 | 1,125 | 3,578 | ||||||||||||||||||||||||||||||
| 7,526 | 3,099 | 2/1/2021 | $ | 17.35 | 1/31/2031 | — | — | |||||||||||||||||||||||||
| 2/1/2021 | 435 | 1,383 | ||||||||||||||||||||||||||||||
| 12,395 | 8,855 | 8/1/2021 | $ | 7.60 | 7/31/2031 | — | — | |||||||||||||||||||||||||
| 8/1/2021 | 1,875 | 5,963 | ||||||||||||||||||||||||||||||
| 5,500 | 6,500 | 2/1/2022 | $ | 10.02 | 1/31/2032 | — | — | |||||||||||||||||||||||||
| 2/1/2022 | 750 | 2,385 | ||||||||||||||||||||||||||||||
| 1,250 | 4,750 | 2/1/2023 | $ | 3.52 | 1/31/2033 | |||||||||||||||||||||||||||
| 2,000 | (5) | 2/1/2023 | $ | 3.52 | 1/31/2025 | |||||||||||||||||||||||||||
| 2,000 | (6) | 2/1/2023 | $ | 3.52 | 1/31/2025 | |||||||||||||||||||||||||||
| 2,000 | (7) | 2/1/2023 | $ | 3.52 | 1/31/2025 | |||||||||||||||||||||||||||
| 2/1/2023 | 1,000 | 3,180 | ||||||||||||||||||||||||||||||
| (1) | Unless otherwise indicated, these options have a 10-year term and vest in substantially equal monthly installments over a four-year period, subject to the recipient's continued employment with us through such vesting dates. | |||||||||||||||||||||||||||||||
| (2) | This column shows options that were unvested as of December 31, 2023. | |||||||||||||||||||||||||||||||
| (3) | Unless otherwise indicated, these RSUs vest annually over a four-year period, with 25% vesting on each of the first, second, third and fourth anniversaries of grant date, subject to the recipient's continued employment with us through such vesting dates. | |||||||||||||||||||||||||||||||
| (4) | Calculated by multiplying the number of RSUs by $3.18, the closing market price of our common stock on December 31, 2023. | |||||||||||||||||||||||||||||||
| (5) | These options will vest upon an increase of closing share price of the Company’s common stock on the Nasdaq Stock Market by at least 50% above exercise price of the stock options, when measured over 30 consecutive calendar days (must occur within two years of the Date of Grant or such shares will be forfeited). | |||||||||||||||||||||||||||||||
| (6) | These options will vest upon an increase of closing share price of the Company’s common stock on the Nasdaq Stock Market by at least 100% above exercise price of the stock options, when measured over 30 consecutive calendar days (must occur within two years of the Date of Grant or such shares will be forfeited). | |||||||||||||||||||||||||||||||
| (7) | These options will vest upon an increase of closing share price of the Company’s common stock on the Nasdaq Stock Market by at least 150% above exercise price of the stock options, when measured over 30 consecutive calendar days (must occur within two years of the Date of Grant or such shares will be forfeited). | |||||||||||||||||||||||||||||||
| (8) | These options vested following enrollment reaching 50% of total patients enrolled for the LUM-201 trial as defined by the Board. | |||||||||||||||||||||||||||||||
| (9) | These options vested following completion of the LUM-201 trial as defined by the Board. | |||||||||||||||||||||||||||||||
| (10) | These options were granted in connection with the forfeiture of previously held options under the stock option exchange program described in more detail and approved by stockholders at our 2019 Annual Meeting. | |||||||||||||||||||||||||||||||
Potential Payments Upon Termination or Change in Control
Under our 2009 Equity Incentive2019 Plan, the vesting of stock options granted to our employees and officers may be accelerated in connection with specified corporate transactions and change in control transactions. Other than as set forth in the tables below, none of our other option grants provide for acceleration of vesting of any options in connection with such a transaction, except for certain options originally granted under our 2000 Equity Incentive Plan that may vest upon a change in control if the acquirer does not assume outstanding option grants. In addition, under our 2010 Non-Employee Directors’ Stock Award Plan, in the event of a change in control, 100% of the shares subject to each Director’sdirector’s options will vest.
Under the terms of employment agreements with certain of our named executive officers in effect as of December 31, 2016,2020, if we terminate such named executive officer’s employment for “cause” or such named executive officer resigns without “good reason,” such named executive officer is entitled to the following: (i) any salary earned but unpaid prior to termination; (ii) any benefits accrued prior to termination; (iii) all accrued but unused vacation; and (iv) any business expenses that were incurred but not reimbursed as of the date of termination (collectively, the “Accrued Obligations”). Following such termination, vesting of such named executive officer’s then outstanding stock options shall cease on the date of such termination.
Under the terms of employment agreements with such named executive officers, if we terminate such named executive officer’s employment without cause or such named executive officer resigns with good reason (other than in connection with a change in control), and in each case such named executive officer signs a general release and written acknowledgment of his or
32
her continuing obligations under his or her confidentiality and inventions assignment agreement with us, such named executive officer is entitled to the following: (i) payment of the Accrued Obligations; (ii) depending on the named executive officer and as described in the tables below, the equivalent of 24, 1812 or 6 months of such named executive officer’s base salary as in effect immediately prior to the termination date, payable on the same basis and at the same time as previously paid and subject to employment tax withholdings and deductions; (iii) for certain of the named executive officers and as described in the tables below, a bonus payout equal to the most recent annual bonus paid to the named executive officer or a portion thereof; and (iv) depending on the named executive officer and as described in the tables below, payment of such named executive officer’s COBRA premiums for 24, 18, 12 or 6 months to be paid in order for such named executive officer to maintain medical insurance coverage that is substantially equivalent to that which such named executive officer received immediately prior to the termination payment of premiums for his or her group health insurance.insurance and (v) 12 months accelerated vesting of such named executive officer’s equity compensation awards (so such executive becomes vested in the portion of such awards that would have become vested if executive remained employed for 365 days after the termination date) and depending on the named executive officer and as described in the tables below, extension of the window to exercise such options for up to 12 months. In the event that such named executive officer breaches his or her confidentiality, non-compete or non-solicitation obligations under his or her confidentiality and inventions assignment agreement with us, the payments described above, except for the Accrued Obligations, shall cease, and we shall have no further obligations to such named executive officer with respect thereto. Our obligation to pay such named executive officer’s COBRA premiums ceases upon such named executive officer’s eligibility for comparable coverage provided by a new employer.
Under the terms of the employment agreements with the named executive officers in effect as of December 31, 2016,2021, if we (or any surviving or acquiring corporation) terminate a named executive officer’s employment without cause or a named
executive officer resigns with good reason within one month prior to or 13 months following the effective date of a change in control (either constituting a “Change of Control Termination”), and in each case such named executive officer signs a general release and written acknowledgment of his or her continuing obligations under his or her confidentiality and inventions assignment agreement with us, such named executive officer is entitled to the following: (i) payment of the Accrued Obligations; (ii) depending on the named executive officer and as described in the tables below, the equivalent of 24, 18 or 12 months of such named executive officer’s base salary as in effect immediately prior to the termination date, payable on the same basis and at the same time as previously paid and subject to employment tax withholdings and deductions; (iii) depending on the named executive officer and as described in the tables below, a bonus payout equal to two, one and one-half or one times the most recent annual cash bonus paid to the named executive officer; (vi)(iv) depending on the named executive officer as described in the tables below, payment of such named executive officer’s COBRA premiums for 24, 18 or 12 months to be paid in order for such named executive officer to maintain medical insurance coverage that is substantially equivalent to that which such named executive officer received immediately prior to the termination payment of premiums for his or her group health insurance; and (v) we will vest 100% of the shares subject to such named executive officer’s equity compensation awards and such vesting shall occur upon the occurrence of the change of control in the case of a Change of Control Termination occurring prior to the change in control or upon termination in the case of a Change of Control Termination occurring after the change of control. If a named executive officer breaches his or her confidentiality, non-compete or non-solicitation obligations under his or her confidentiality and inventions assignment agreement with us, the payments described above, except for the Accrued Obligations, shall cease, and we shall have no further obligations to such named executive officer with respect thereto. Our obligation to pay such named executive officer’s COBRA premiums ceases upon such named executive officer’s eligibility for comparable coverage provided by a new employer.
The following tables reflect the estimated potential payments that would be payable to each named executive officer, upon a termination or change in control of us under the terms of his or her employment agreement in effect as of December 31, 2016.2022. The amounts shown below reflect only the additional payments or benefits that each named executive officer would have received upon the occurrence of the respective triggering events listed below, but they do not include the value of payments or benefits that would have been earned, or any amounts associated with equity awards that would have vested, absent the triggering event. For purposes of calculating the potential payments set forth in the tables below, we have assumed that (i) the date of termination was December 31, 20162023 and (ii) the stock price was $10.28,$3.18, which was the per share closing price of our common stock on the NASDAQ Global Market on December 31, 2016.
2023.
33
| Charles J. Link, Jr., M.D. | Termination For Just Cause or Resignation Without Good Reason | Termination Without Just Cause or Resignation With Good Reason | Termination Without Just Cause or Resignation With Good Reason (in connection with a Change in Control) | ||||||||||
| Cash Payments | |||||||||||||
| Cash Severance | — | $ | 1,550,765 | (1) | $ | 1,781,730 | (2) | ||||||
| Long-Term Incentives | |||||||||||||
| Stock Options (Unvested and Accelerated) | — | — | $ | 944,619 | (3) | ||||||||
| Benefits and Perquisites | |||||||||||||
| Accrued Obligations | $ | 299,493 | (4) | $ | 299,493 | (4) | $ | 299,493 | (4) | ||||
| Benefits Continuation | — | $ | 29,610 | (5) | $ | 29,610 | (5) | ||||||
| Total Payments Upon Termination | $ | 299,493 | $ | 1,879,868 | $ | 3,055,452 | |||||||
| Richard J. Hawkins | Termination For Just Cause or Resignation Without Good Reason | Termination Without Just Cause or Resignation With Good Reason | Termination Without Just Cause or Resignation With Good Reason (in connection with a Change in Control) | |||||||||||||||||
| Cash Payments | ||||||||||||||||||||
| Cash Severance | — | $ | 806,935 | (1) | $ | 1,613,870 | (2) | |||||||||||||
| Long-Term Incentives | ||||||||||||||||||||
| RSUs and Stock Options (Unvested and Accelerated) | — | 48,622 | (3)(4) | 48,622 | (3)(4) | |||||||||||||||
| Benefits and Perquisites | ||||||||||||||||||||
| Accrued Obligations | 100,839 | (5) | 100,839 | (5) | 100,839 | (5) | ||||||||||||||
| Benefits Continuation | — | 20,306 | (6) | 40,613 | (7) | |||||||||||||||
| Total Payments Upon Termination | $ | 100,839 | $ | 976,702 | $ | 1,803,944 | ||||||||||||||
| (1) | Amount represents 12 months of his base salary in effect as of January 1, 2023 and an amount equal to his most recent annual bonus. | |||||
| Amount represents 24 months of his base salary | ||||||
| Amount represents the | ||||||
| (4) | Amount represents the value of in-the-money unvested stock options as of December 31, | |||||
| Amount represents | ||||||
| (6) | Amount represents 12 months of COBRA premiums. | |||||
| Amount represents 24 months of COBRA premiums. | ||||||
| Nicholas N. Vahanian, M.D. | Termination For Just Cause or Resignation Without Good Reason | Termination Without Just Cause or Resignation With Good Reason | Termination Without Just Cause or Resignation With Good Reason (in connection with a Change in Control) | ||||||||||
| Cash Payments | |||||||||||||
| Cash Severance | — | $ | 1,005,025 | (1) | $ | 1,256,281 | (2) | ||||||
| Long-Term Incentives | |||||||||||||
| Stock Options (Unvested and Accelerated) | — | — | $ | 572,658 | (3) | ||||||||
| Benefits and Perquisites | |||||||||||||
| Accrued Obligations | $ | 218,786 | (4) | $ | 218,786 | (4) | $ | 218,786 | (4) | ||||
| Benefits Continuation | — | $ | 22,207 | (5) | $ | 25,908 | (6) | ||||||
| Total Payments Upon Termination | $ | 218,786 | $ | 1,246,018 | $ | 2,073,633 | |||||||
| John C. McKew | Termination For Just Cause or Resignation Without Good Reason | Termination Without Just Cause or Resignation With Good Reason | Termination Without Just Cause or Resignation With Good Reason (in connection with a Change in Control) | |||||||||||||||||
| Cash Payments | ||||||||||||||||||||
| Cash Severance | — | $ | 535,500 | (1) | $ | 1,084,388 | (2) | |||||||||||||
| Long-Term Incentives | ||||||||||||||||||||
| RSUs and Stock Options (Unvested and Accelerated) | — | 53,787 | (3)(4) | 53,787 | (3)(4) | |||||||||||||||
| Benefits and Perquisites | ||||||||||||||||||||
| Accrued Obligations | 92,683 | (5) | 92,683 | (5) | 92,683 | (5) | ||||||||||||||
| Benefits Continuation | — | 20,306 | (6) | 30,459 | (7) | |||||||||||||||
| Total Payments Upon Termination | $ | 92,683 | $ | 702,276 | $ | 1,261,317 | ||||||||||||||
| (1) | Amount represents 12 months of his base salary in effect as of January 1, 2023. | |||||
| Amount represents 18 months of his base salary | ||||||
| Amount represents the | ||||||
| (4) | Amount represents the value of in-the-money unvested stock options as of December 31, | |||||
| Amount represents | ||||||
| (6) | Amount represents 12 months of COBRA premiums. | |||||
| Amount represents 18 months of COBRA premiums. | ||||||
34
| John B. Henneman, III | Termination For Just Cause or Resignation Without Good Reason | Termination Without Just Cause or Resignation With Good Reason | Termination Without Just Cause or Resignation With Good Reason (in connection with a Change in Control) | ||||||||||
| Cash Payments | |||||||||||||
| Cash Severance | — | $ | 423,600 | (1) | $ | 762,480 | (2) | ||||||
| Long-Term Incentives | |||||||||||||
| Stock Options (Unvested and Accelerated) | — | — | $ | 391,627 | (3) | ||||||||
| Benefits and Perquisites | |||||||||||||
| Accrued Obligations | $ | 91,237 | (4) | $ | 91,237 | (4) | $ | 91,237 | (4) | ||||
| Benefits Continuation | — | $ | 14,805 | (5) | $ | 22,207 | (6) | ||||||
| Total Payments Upon Termination | $ | 91,237 | $ | 529,642 | $ | 1,267,551 | |||||||
| Lori Lawley | Termination For Just Cause or Resignation Without Good Reason | Termination Without Just Cause or Resignation With Good Reason | Termination Without Just Cause or Resignation With Good Reason (in connection with a Change in Control) | |||||||||||||||||
| Cash Payments | ||||||||||||||||||||
| Cash Severance | — | $ | 391,000 | (1) | $ | 500,480 | (2) | |||||||||||||
| Long-Term Incentives | ||||||||||||||||||||
| RSUs and Stock Options (Unvested and Accelerated) | — | 16,488 | (3)(4) | 16,488 | (3)(4) | |||||||||||||||
| Benefits and Perquisites | ||||||||||||||||||||
| Accrued Obligations | 67,673 | (5) | 67,673 | (5) | 67,673 | (5) | ||||||||||||||
Benefits Continuation(6) | — | 12,653 | (6) | 12,653 | (6) | |||||||||||||||
| Total Payments Upon Termination | $ | 67,673 | $ | 487,814 | $ | 597,294 | ||||||||||||||
| Amount represents 12 months of | |||||
| Amount represents | |||||
| Amount represents the | |||||
| (4) | Amount represents the value of in-the-money unvested stock options as of December 31, | ||||
| Amount represents | |||||
| Brian Wiley | Termination For Just Cause or Resignation Without Good Reason | Termination Without Just Cause or Resignation With Good Reason | Termination Without Just Cause or Resignation With Good Reason (in connection with a Change in Control) | ||||||||||
| Cash Payments | |||||||||||||
| Cash Severance | — | $ | 185,350 | (1) | $ | 435,573 | (2) | ||||||
| Long-Term Incentives | |||||||||||||
| Stock Options (Unvested and Accelerated) | — | — | $ | 151,959 | (3) | ||||||||
| Benefits and Perquisites | |||||||||||||
| Accrued Obligations | $ | 79,843 | (4) | $ | 79,843 | (4) | $ | 79,843 | (4) | ||||
| Benefits Continuation | — | $ | 7,402 | (5) | $ | 14,805 | (6) | ||||||
| Total Payments Upon Termination | $ | 79,843 | $ | 272,595 | $ | 682,180 | |||||||
| Amount represents 12 months of COBRA premiums. | ||||
Pay Versus Performance Disclosure
In accordance with rules adopted by the Securities and Exchange Commission pursuant to the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010, we provide the following disclosure regarding executive compensation for our principal executive officer (“PEO”) and Non-PEO named executive officers (“NEOs”) and Company performance for the fiscal years listed below. The Compensation Committee did not consider the pay versus performance disclosure below in making its pay decisions for any of the years shown.
| Value of Initial Fixed $100 Investment Based On: | ||||||||||||||||||||
| Fiscal Year | Summary Compensation Table for PEO(1) | Compensation Actually Paid to PEO(2)(3) | Average Summary Compensation Table Totals for non-PEO NEOs(1) | Average Compensation Actually Paid to non-PEO NEOs(2)(3) | Total Shareholder Return | Net Income (thousands) | ||||||||||||||
| (a) | (b) | (c) | (d) | (e) | (f) | (g) | ||||||||||||||
| 2023 | $952,869 | $896,991 | $737,283 | $710,330 | $8.91 | $(34,034) | ||||||||||||||
| 2022 | $1,272,873 | $964,542 | $875,104 | $714,372 | $10.11 | $(31,062) | ||||||||||||||
| 2021 | $1,353,439 | $(2,577,575) | $1,106,922 | $(353,414) | $19.41 | $(30,430) | ||||||||||||||
35
| Carl Langren | Termination For Just Cause or Resignation Without Good Reason | Termination Without Just Cause or Resignation With Good Reason | Termination Without Just Cause or Resignation With Good Reason (in connection with a Change in Control) | ||||||||||
| Cash Payments | |||||||||||||
| Cash Severance | — | $ | 138,750 | (1) | $ | 319,125 | (2) | ||||||
| Long-Term Incentives | |||||||||||||
| Stock Options (Unvested and Accelerated) | — | — | $ | 100,919 | (3) | ||||||||
| Benefits and Perquisites | |||||||||||||
| Accrued Obligations | $ | 24,548 | (4) | $ | 24,548 | (4) | $ | 24,548 | (4) | ||||
| Benefits Continuation | — | $ | 7,402 | (5) | $ | 14,805 | (6) | ||||||
| Total Payments Upon Termination | $ | 24,548 | $ | 170,700 | $ | 459,397 | |||||||
| (1) | The PEO for 2023, 2022 and 2021 is Richard J. Hawkins. The Non-PEO NEOs for whom the average compensation is presented in this table for 2023, 2022 and 2021 are John McKew and Lori Lawley. | |||||
| (3) | Compensation Actually Paid reflects the exclusions and inclusions of certain amounts for the PEO and the Non-PEO NEOs as set forth below. Equity values are calculated in accordance with FASB ASC Topic 718. | |||||
| PEO | |||||
| Prior FYE | 12/31/22 | ||||
| Current FYE | 12/31/23 | ||||
| Fiscal Year | 2023 | ||||
| SCT Total | $ | 952,869 | |||
| Minus Change in Pension Value and Above Market Non-Qualified Deferred Compensation | $ | — | |||
| Minus Grant Date Fair Value of Options Awards and Stock Awards Granted in Fiscal Year | $ | (98,517) | |||
| Plus Fair Value at Fiscal Year-End of Outstanding and Unvested Option Awards and Stock Awards Granted in Fiscal Year | $ | 66,018 | |||
| Plus Change in Fair Value of Outstanding and Unvested Options Awards and Stock Awards Granted in Prior Fiscal Years | $ | (19,117) | |||
| Plus Fair Value at Vesting of Option Awards and Stock Awards Granted in Fiscal Year That Vested During Fiscal Year | $ | 9,423 | |||
| Plus Change in Fair Value as of Vesting Date of Option Awards and Stock Awards Granted in Prior Fiscal Years For Which Applicable Vesting Conditions Were Satisfied During Fiscal Year | $ | (13,685) | |||
| Minus Fair Value as of Prior Fiscal Year-End of Option Awards and Stock Awards Granted in Prior Fiscal Years That Failed to Meet Applicable Vesting Conditions During Fiscal Year | $ | — | |||
| Plus Value of Dividends or other Earnings Paid on Stock or Option Awards not Otherwise Reflected in Fair Value or Total Compensation | $ | — | |||
| Compensation Actually Paid | $ | 896,991 | |||
| NEOs | |||||
| Prior FYE | 12/31/22 | ||||
| Current FYE | 12/31/23 | ||||
| Fiscal Year | 2023 | ||||
| SCT Total | $ | 737,283 | |||
| Minus Change in Pension Value and Above Market Non-Qualified Deferred Compensation | $ | — | |||
| Minus Grant Date Fair Value of Options Awards and Stock Awards Granted in Fiscal Year | $ | (37,978) | |||
| Plus Fair Value at Fiscal Year-End of Outstanding and Unvested Option Awards and Stock Awards Granted in Fiscal Year | $ | 25,220 | |||
| Plus Change in Fair Value of Outstanding and Unvested Options Awards and Stock Awards Granted in Prior Fiscal Years | $ | (11,439) | |||
| Plus Fair Value at Vesting of Option Awards and Stock Awards Granted in Fiscal Year That Vested During Fiscal Year | $ | 3,726 | |||
| Plus Change in Fair Value as of Vesting Date of Option Awards and Stock Awards Granted in Prior Fiscal Years For Which Applicable Vesting Conditions Were Satisfied During Fiscal Year | $ | (6,483) | |||
| Minus Fair Value as of Prior Fiscal Year-End of Option Awards and Stock Awards Granted in Prior Fiscal Years That Failed to Meet Applicable Vesting Conditions During Fiscal Year | $ | — | |||
| Plus Value of Dividends or other Earnings Paid on Stock or Option Awards not Otherwise Reflected in Fair Value or Total Compensation | $ | — | |||
| Compensation Actually Paid | $ | 710,330 | |||
36
Description of Relationship Between PEO and Non-PEO NEO Compensation Actually Paid and Company Total Shareholder Return ("TSR")
The following chart sets forth the relationship between Compensation Actually Paid ("CAP") to our PEO, the average of Compensation Actually Paid to our Non-CEO NEOs, and the Company's TSR over the three most recently completed fiscal years.
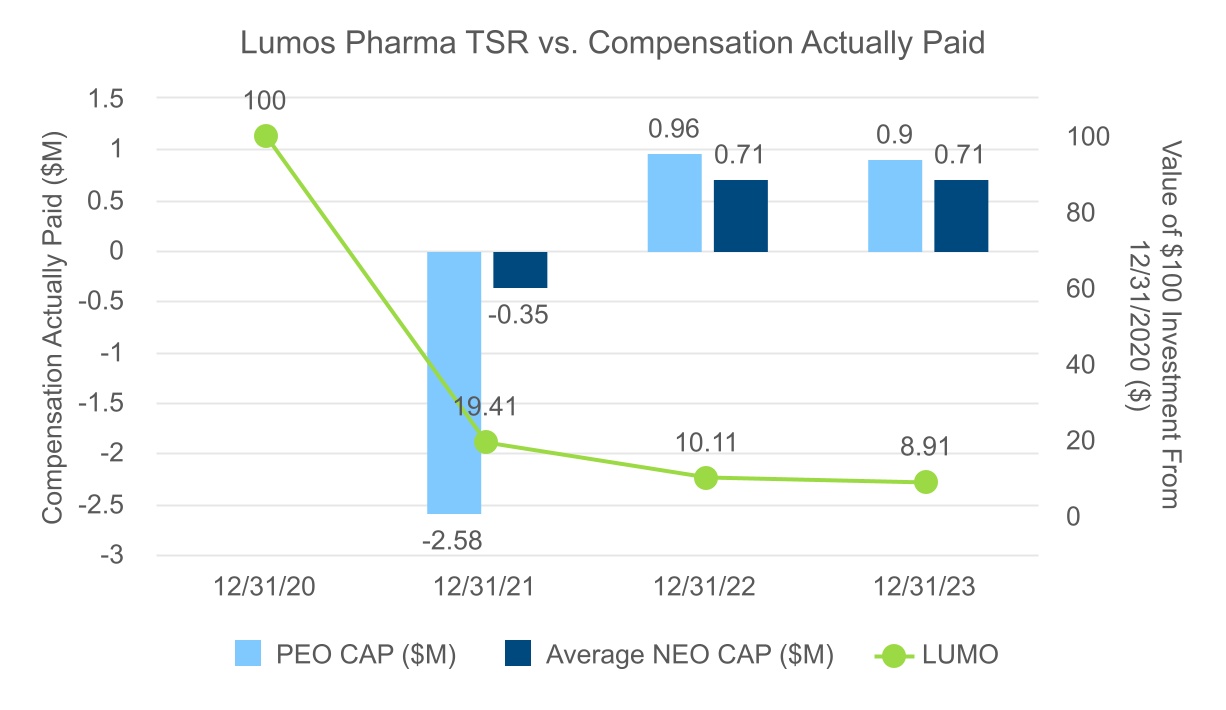
37
Description of Relationship Between PEO and Non-PEO NEO Compensation Actually Paid and Company Net Loss
The following chart sets forth the relationship between Compensation Actually Paid to our PEO, the average of Compensation Actually Paid to our Non-PEO NEOs, and the Company’s net loss over the three most recently completed fiscal years.

38
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
We have not been a party since January 1, 20132023 to any transactions, in which the amount involved in the transaction exceeds $120,000 (which is less than 1% of the average of our total assets at the end of the last two completed fiscal years), and in which any of our directors, executive officers or to our knowledge, beneficial owners of more than 5% of our capital stock had or will have a direct or indirect material interest, other than compensation, termination and change in control arrangements, which are described in more detail in the Compensation Discussion and Analysissection entitled "Executive Compensation" beginning on page 2224 of this proxy statement.
Policies and Procedures for Related Person Transactions
Our Board has adopted written policies and procedures for the review of any transaction, arrangement or relationship in which we are a participant, the amount involved exceeds $120,000 and one of our executive officers, directors, director nominees or 5% stockholders (or their immediate family members), each of whom we refer to as a “related"related person,”" has a direct or indirect material interest.
If a related person proposes to enter into such a transaction, arrangement or relationship, which we refer to as a “related"related person transaction,”" the related person must report the proposed related person transaction to our Audit Committee. The policy calls for the proposed related person transaction to be reviewed and, if deemed appropriate, approved by our Audit Committee. Whenever practicable, the reporting, review and approval will occur prior to entry into the transaction. If advance review and approval is not practicable, our Audit Committee will review, and, in its discretion, may ratify the related person transaction. Any related person transactions that are ongoing in nature will be reviewed annually.
A related person transaction reviewed under the policy will be considered approved or ratified if it is authorized by our Audit Committee after full disclosure of the related person’s interest in the transaction. As appropriate for the circumstances, our Audit Committee will review and consider:
•the related person’sperson's interest in the related person transaction;
•the approximate dollar value of the amount involved in the related person transaction;
•the approximate dollar value of the amount of the related person’sperson's interest in the transaction without regard to the amount of any profit or loss;
•whether the transaction was undertaken in the ordinary course of our business;
•whether the terms of the transaction are no less favorable to us than terms that could have been reached with an unaffiliated third party;
•the purpose of, and the potential benefits to us of, the transaction; and
•any other information regarding the related person transaction or the related person in the context of the proposed transaction that would be material to investors in light of the circumstances of the particular transaction.
Our Audit Committee may approve or ratify the transaction only if the committeeAudit Committee determines that, under all of the circumstances, the transaction is in, or is not inconsistent with, our best interests. Our Audit Committee may impose any conditions on the related person transaction that it deems appropriate.
In addition to the transactions that are excluded by the instructions to the SEC’s related person transaction disclosure rule, our Board has determined that the following transactions do not create a material direct or indirect interest on behalf of related persons and, therefore, are not related person transactions for purposes of this policy:
•interests arising solely from the related person’s position as an executive officer of another entity (whether or not the person is also a director of such entity), that is a participant in the transaction, where (a) the related person and all other related persons own in the aggregate less than a 10% equity interest in such entity, (b) the related person and his or her immediate family members are not involved in the negotiation of the terms of the transaction and do not receive any special benefits as a result of the transaction or (c) the amount involved in the transaction equals less than the greater of $200,000 or 5% of the annual consolidated gross revenues of our receiving payment under the transaction; and
•a transaction that is specifically contemplated by provisions of our certificate of incorporation or bylaws.
The policy provides that transactions involving compensation of executive officers shall be reviewed and approved by our Compensation Committee in the manner specified in its charter.
39
Limitation of Liability and Indemnification
Our amended and restated bylawsBylaws require us to indemnify our directors to the fullest extent not prohibited by law and permit us to indemnify our officers, employees and other agents as set forth under Delaware law. We will indemnify any such person in connection with a proceeding initiated by such person only if such indemnification is expressly required by law, the proceeding was authorized by our Board, the indemnification is provided by us, in our sole discretion, pursuant to the Delaware General Corporation Law or other applicable law or is otherwise expressly required by our amended and restated bylaws.Bylaws. Section 145 of the Delaware General Corporation Law permits indemnification of officers, directors and other agents under specified circumstances and subject to specified limitations. Delaware law also permits a corporation not to hold its directors personally liable for monetary damages for breach of their fiduciary duties as directors, except for liability for:
•breach of their duty of loyalty to the corporation or its stockholders;
•acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law;
•unlawful payments of dividends or unlawful stock repurchases or redemptions; and
•any transaction from which the director derived an improper personal benefit.
These limitations of liability do not affect the availability of equitable remedies such as injunctive relief or rescission.
Our amended and restated bylawsBylaws also permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in this capacity. We have obtained directors’ and officers’ liability insurance to cover certain liabilities described above.
We have entered into indemnity agreements with each of our directors that require us to indemnify such persons against any and all expenses, including attorneys’ fees, witness fees, judgments, fines, settlements and other amounts incurred, including expenses of a derivative action, in connection with any action, suit or proceeding or alternative dispute resolution mechanism, inquiry hearing or investigation, whether threatened, pending or completed, to which any such person may be made a party by reason of the fact that such person is or was one of our directors, officers or employees, provided that such person’s conduct did not constitute a breach of his or her duty of loyalty to us or our stockholders, and was not an act or omission not in good faith or which involved intentional misconduct or a knowing violation of laws. The indemnity agreements also set forth procedures that will apply in the event of a claim for indemnification thereunder. We believe that these provisions and agreements are necessary to attract and retain qualified persons as our directors.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted by directors, executive officers or persons controlling us, we have been informed that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
OTHER INFORMATION
Stockholder Communications with Our Board of Directors
Our Board has adopted a formal process by which stockholders may communicate with our Board or any of its directors. This information is available on our website at www.newlinkgenetics.comwww.lumos-pharma.com in the “Investors & Media - Corporate Governance - Contact the Board” section.
Stockholder Proposals and Nominations of Directors
Stockholders who wish to submit a proposal for our 20182025 Annual Meeting of Stockholders must submit any such proposal by November 29, 2017,December 13, 2024, to Corporate Secretary, NewLink Genetics CorporationLumos Pharma, Inc., 2503 South Loop Drive, Ames, Iowa 500104200 Marathon Boulevard #200, Austin, TX 78756. If you wish to submit a director nomination or a proposal at next year’s annual meeting that is not to be included in next year’s proxy materials, you must do so by no later than the close of business on February 12, 2018,March 2, 2025, nor earlier than the close of business on
January 12, 2018,31, 2025, and you must comply with the requirements of Section 5(b) in the our Bylaws, including submitting written notice to our Corporate Secretary as set forth above. Also, if you intend to solicit proxies in support of director nominees other than the Company’s nominees, then we must receive notice providing the information required by Rule 14a-19 of the Exchange Act postmarked no later than April 1, 2025, and you must comply with the applicable requirements in our Bylaws.
40
The Company has not yet selected the date of the annual meeting of stockholders for next year but is considering holdingcurrently plans to hold the meeting in May 2018.2025. If the date of the 20182025 annual meeting is advanced or delayed more than 30 days before or after the anniversary of the date of this Annual Meeting, notice by the stockholder to be timely must be so received notno earlier than the close of business on the 120th day prior to such 20182025 annual meeting and not later than the close of business on the later of the 90th day prior to such 20182025 annual meeting or the 10th day following the day on which public announcement of the date of such meeting is first made. Submissions must include the full name of the proposed nominee, a description of the proposed nominee’s business experience for at least the previous five years, complete biographical information, a description of the proposed nominee’s qualifications as a director, a representation that the nominating stockholder is a beneficial or record holder of our common stock and such other information as is required under Section 5(b) of our Bylaws. Any such submission must be accompanied by the written consent of the proposed nominee to be named as a nominee and to serve as a director if elected.
Householding of Proxy Materials
The SEC has adopted rules that permit companies and intermediaries (e.g., brokers) to satisfy the delivery requirements for NoticesNotice of Internet Availability of Proxy Materials or other Annual Meeting materialswith respect to two or more stockholders sharing the same address by delivering a single Notice of Internet Availability of Proxy Materials or other Annual Meeting materialsaddressed to those stockholders. This process, which is commonly referred to as “householding,” potentially means extra convenience for stockholders and cost savings for companies.
This year, a number of brokers with account holders who are NewLink Genetics CorporationLumos stockholders will be “householding” our proxy materials. A single Notice of Internet Availability of Proxy Materials will be delivered to multiple stockholders sharing an address unless contrary instructions have been received from the affected stockholders. Once you have received notice from your broker that they will be “householding” communications to your address, “householding” will continue until you are notified otherwise or until you revoke your consent. If, at any time, you no longer wish to participate in “householding” and would prefer to receive a separate Notice of Internet Availability of Proxy Materials, please notify your broker or NewLink Genetics Corporation.Lumos. Direct your written request to Corporate Secretary, NewLink Genetics Corporation, 2503 South Loop Drive, Ames, IA 50010Lumos Pharma, Inc., 4200 Marathon Boulevard #200, Austin, TX 78756 or contact our Corporate Secretary at (515)598-2561.(512) 215-2630. We undertake to promptly deliver, upon written or oral request, a separate copy of our Notice of Internet Availability of Proxy Materials to stockholders at a shared address to which a single copy of such documents was delivered. Stockholders who currently receive multiple copies of the NoticesNotice of Internet Availability of Proxy Materials at their addresses and would like to request “householding” of their communications should contact their brokers.
41
OTHER MATTERS
Our Board knows of no other matters that will be presented for consideration at the Annual Meeting. If any other matters are properly brought before the meeting, it is the intention of the persons named in the accompanying proxy to vote on such matters in accordance with their best judgment.
By Order of the Board of Directors
/s/ John B. Henneman, IIILori Lawley
A copy of our Annual Report to the Securities and Exchange Commission on Form 10-K for the fiscal year ended December 31, 20162023 is available without charge upon written request to: Corporate Secretary, NewLink Genetics Corporation, 2503 South Loop Drive, Suite 5100, Ames, IA 50010.


Lumos Pharma, Inc., 4200 Marathon Boulevard #200, Austin TX 78756.
42